Basic to Clinical
A New Compound to Activate the μ Opioid Receptor.
Structure-based discovery of opioid analgesics with reduced side effects.
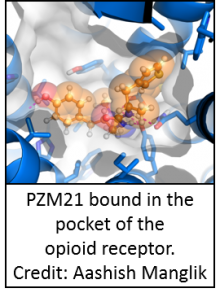
Opioids are a group of substances that bind opioid receptors in the body. Though opioids have been used for centuries to treat pain, the side effects of opioid use are extreme ranging from constipation to potentially fatal respiratory depression. Moreover, opioids are highly addictive and prone to misuse which has been estimated to cost the US $78 billion annually. Opioid receptors can be classified as μ, δ, κ or nociception types. Recent work has uncovered that the pain relieving effect of opioids may occur through a specific interaction with the μ opioid receptor, whereby, a molecular switch known as a G-protein is activated. In contrast, the negative effects of opioids were suggested to take place when opioids interact with the μ opioid receptor and activate β-arrestin, a signaling molecule that usually blocks G-protein coupled receptors. The authors of this study hypothesized that if a molecule could interact with the μ opioid receptor and activate the G-protein but not the β-arrestin pathway it would provide the pain relief of opioids, without the negative side effects. To test this, the authors first set out to find a molecule that had such properties. Based off the known crystal structure of the μ opioid receptor, the authors used computer models to screen over 3 million compounds for docking to the μ opioid receptor. The authors then manually screened the top 2500 molecules that docked best to the receptor and selected 23 novel molecules that were predicted to interact with the receptor in new ways. The authors then synthesized modified versions of some of these compounds to enhance binding to the μ opioid receptor and arrived at a compound that strongly bound the μ opioid receptor (but not the κ opioid receptor) and activated the G-protein signaling pathway but not the β-arrestin pathway. This compound was then further modified to create a new compound, called PZM21, which had higher affinity and potency at the μ opioid receptor. This compound was confirmed to be selective for the μ opioid receptor and preferentially activated the G-protein signaling with little β-arrestin recruitment. Moreover, when administered to animals, PZM21 produced pain relief in mice, with much lower levels of side effects. Finally, PZM21 also did not appear to impact the dopaminergic reward circuits (which have been implicated in opioid addiction). Therefore, beginning with a computational docking screen, the authors were able to synthesize a novel μ opioid receptor ligand that specifically triggers the pain relieving effects, while avoiding the negative side effects associated with traditional opioids.
Disparities
A 12-Year Study to Examine Sociodemographic Disparities in Pain
Sociodemographic disparities in chronic pain, based on 12-year longitudinal data.
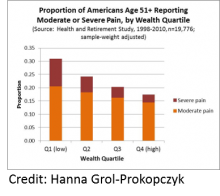
It is becoming apparent that chronic pain is a serious public health problem. In order to understand chronic pain it is imperative that we have a clear view of how many people have chronic pain in the US population and how sociodemographic variables impact the effect of chronic pain. To date, most estimates addressing the above issues are taken from cross-sectional studies, where a population is assessed at a single timepoint with little to no consideration for the role of measurement bias in their results. The author of this study points out that 2 of the patterns which have emerged from these cross-sectional studies, (1) the likelihood of pain rising up to age 60 then staying steady and (2) whites report pain more frequently than non-Hispanic blacks or Hispanics, are unexpected. The author of this report expanded on these cross-sectional studies by utilizing a study design that follows a population over time to assess sociodemographic disparities a in noncancer pain and assess measurement bias. The report used data from an National Institute on Aging sponsored study (The Health and Retirement Study) that followed almost 20 000 US adults over 50 years of age for 12 years. What the author found was that pain prevalence increased over the 12 years of this study (1998-2010) and in agreement with previous studies women and people of lower education/wealth reported higher pain than men and people with higher education/wealth, respectively. The authors also found that contrary to previous findings of pain plateauing at 60 years of age pain increased with time, across all age groups. The author explained this disagreement with previous studies by determining that pain predicted death, which indicates that in a cross-sectional study the pain curve appears to flatten because the people with the most pain are more likely to have died and not participate in the survey. One surprising finding was that when controlling for socioeconomic status there was no difference in pain levels observed between whites and Hispanics, while African-Americans reported lower pain levels than whites. Next the authors assessed differences in pain reporting and determined, critically, that the groups found to be experiencing the most pain (women, the less wealthy and the less educated) reported pain differently than their respective counterparts, meaning that these pain disparities may be underestimated when reporting bias is not taken into account. Overall, this report provides key guidance for public health researchers by highlighting important disparities in chronic pain and determining sources of measurement bias that should be addressed in future studies. Critically, this work also implicates pain as a predictor of death, therefore, stressing the importance for making chronic pain research and treatment a priority.
False Beliefs about Pain Perception Predict a Racial Bias in Treatment
Research has reported that black Americans are undertreated for pain as compared to whites and that pain in black Americans is often underestimated, even by physicians. This phenomenon of black patients being under treated for pain has been documented across a number of pain conditions (appendicitis, cancer etc.) and, as the authors of this study point out, could arise by (1) physicians not treating pain because they are concerned patients may not follow the treatment (compliance) or may not have access to the resources required for a treatment or (2) physicians not recognizing pain in black patients. In support of the latter, evidence exists supporting the idea that people believe there is a difference in the amount of pain felt by different races. The authors of this work aimed to assess whether the racial bias in how others perceive pain is related to a person’s beliefs of biological differences between blacks and whites. This study was unique because it assessed how these beliefs may influence racial bias in pain perception in both white lay people and white medical students. The authors found that lay people who had more false beliefs about biological differences between blacks and whites on average rated pain to be lower in black patients as compared to white patients. The authors furthered this study by asking medical students to rate pain and give treatment recommendations for black and white patients. The authors found that medical students that had less false beliefs about biological differences between blacks and whites rated the pain of black patients higher than the pain of white patients, whereas those who had high false beliefs about biological differences rated the pain of black patients to be lower than that of white patients. When the investigators looked at how these medical students were recommending treatment they found that only those with high amounts of false beliefs about biological differences had a racial bias in treatment recommendations. Together this work demonstrated that a significant proportion of white lay people and medical students (including residents) have false beliefs about biological differences between blacks and whites and provides the first evidence that having these beliefs is predictive of a racial bias in pain perception and treatment recommendations.
Pain Mechanisms
Altered Brain Circuits After Nerve Injury Amplify Neuropatic Pain
The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain.
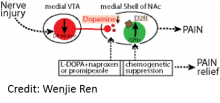
Limbic system neuronal circuits in a region of the brain known as the forebrain are believed to be important for regulating pain. In this paper, authors examined how a key component of these circuits, the medial shell of the nucleus accumbens (msNAc), changes in mice experiencing a nerve injury in their hindpaws. The msNAc is thought to be important in pain processing by integrating nerve inputs from many regions of the brain and previous authors had shown that inactivating the msNAc lowers the hypersensitivity that usually accompanies nerve injury. The authors of this paper furthered this work by investigating the details of how msNAc circuitry is altered during nerve injury. To do this, authors used genetic reporter mice to examine the two known msNAc pathways change after nerve injury. The authors found that one of these pathways, known as the indirect pathway, had altered activity after injury. The authors found that the nerves making up this pathway had less complex dendritic trees (areas that receive input from other neurons) and less frequent mini-neuronal currents (i.e. lower probablility of neuron firing). Moreover, a key neurotransmitter known as dopamine, which inhibits these neurons, was measured to be lower in the msNAc of rats suffering from nerve injury. The authors then probed the role of dopamine as a druggable target to reduce pain in these animals and found that treating mice experiencing nerve injury, orally with a dopamine precursor (L-DOPA) and naproxen (a non steroidal anti-inflammatory drug) reduced the changes in neuronal excitability and morphology. Intriguingly, injured mice treated with L-DOPA and naproxen did not experience hypersensitivity to touch that usually accompanies this injury, indicating that these animals experienced less pain. Furthermore, the authors demonstrated that a compound that can more potently activate the dopamine receptor in the brain was effective at lowering the hypersensitivity induced by nerve injury without the use of naproxen. As a final piece to this work the authors investigated what caused the abnormal firing induced by nerve injury. The authors found that excitatory inputs from the two circuits providing input to the msNAc were altered in nerve injury. Together, this work identified the details about how msNAc is involved in pain after nerve injury and identified a potential target for future treatments for this type of pain.
Altered Gene Expression in Spinal Microglia after Nerve Injury
Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain.
After initial damage to a nerve, chronic neuropathic pain can develop long after the initial insult has healed. This response is thought to be caused by dysfunctional signals that are somehow misinterpreted between the peripheral site where the stimulus is felt and the brain, where the signals are aberrantly translated as pain. One of the midway points in this translation is at the level of the dorsal root ganglia (DRG) in the spinal cord. It has been hypothesized for some time that neuropathy involves activation of microglia, a kind of immune cell of the nervous system, in the spinal cord, but this has not been robustly demonstrated. In this study, researchers from the University of California, San Francisco link all of these pieces together via the molecule colony-stimulating factor 1 (CSF1). The initial study involved looking at what genes were activated in the DRG in response to a peripheral nerve injury. CSF1, a molecule known for inducing differentiation and proliferation of microglia, was one such gene and the scientists subsequently showed that it was transported from the peripheral nerve to the spinal cord after injury. In harmony with this upregulation, the researchers observed that the expression of the CSF1 receptor (CSF1R) was increased specifically in spinal cord microglia after nerve injury, pointing to the importance of this two-step upregulation in communicating peripheral injury to the central nervous system. The researchers then demonstrated that depleting CSF1 from the sensory nerves prevents microglia activation and also mechanical hypersensitivity after nerve injury. The converse was also true in which simply injecting CSF1 intrathecally was enough to cause microglia activation and mechanical hypersensitivity. A separate question in the field had stemmed from the observation that nerve injury causes an overall increase in the total pool of microglia. It was unclear whether this population increase was due to proliferation of resident microglia or an influx of circulating monocytes which then differentiate into microglia. Based on expression profiling of the microglial cells, the researchers concluded that this proliferation was due to self-renewal of the microglia already present in the spinal cord. Tying this to their original observation, they showed that this proliferation is also reliant on the increased expression of CSF1 caused by nerve injury. Lastly, the scientists showed that a similar, but reverse pathway was also activated by injury to motor neurons. This finding indicates that CSF1, and microglia, are key players in mediating signals caused by injury between the periphery, the spinal cord, and the brain. This study provides a new avenue for scientists to explore in treating neuropathic pain.
Spider Toxins Help Uncover a Mechanism of Mechanical Pain
Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain.
Voltage gated sodium (Nav) channels have garnered attention in pain research due to their genetic association with syndromes characterized either by insensitivity to pain or persistent pain. There are several subtypes of these Nav channels that have been well characterized. A particular subtype, the Nav1.1 channel, is known to be associated with a number of neurological disorders but little is known about its role in nociception—the neural response to noxious stimuli. Researchers based at the University of California, San Francisco used a unique method to learn more about this particular Nav1.1 channel. They started by screening more than 100 naturally occurring toxins isolated from spider, scorpion, or centipede venoms in an in vitro preparation of sensory neurons. From this large screen, the scientists isolated two related toxins from tarantula venom, dubbed Hm1a and Hm1b peptides, that caused robust excitation of a subset of the sensory neurons. To understand if these peptides were selective activators of a certain type of Nav channel, the researchers co-treated trigeminal neurons—neurons that innervate the face—with a variety of Nav channel blockers, successfully narrowing down the selectivity to the Nav1.1 channel, and even more specifically to two looped regions of the channel. These initial discoveries allowed the scientists to probe the role the Nav1.1 channel plays in nociception. Injecting subtoxic levels of Hm1a peptide caused mice to display hypersensitivity to a subsequent non-painful mechanical stimulus, but when the Nav1.1 channel was genetically eliminated, this response was attenuated. Importantly, the scientists also connected the Nav1.1 channel to an example of chronic mechanical sensitivity in humans using a rodent model of irritable bowel syndrome (IBS). By testing the responses of gut-nerve preparations in a dish, the researchers observed that the nerves derived from mice with the IBS-like syndrome exhibited elevated mechanosensory responses at baseline and were even more elevated after treatment with Hm1a peptide. This suggests that the Nav1.1 channel could be involved in the hypersensitivity to pain experienced by people who have IBS. The isolation of a tarantula toxin has yielded a very useful tool for understanding more about the Nav1.1 channel. Moreover, it has indicated that blocking Nav1.1 could be a potential treatment option for patients suffering from IBS or perhaps other pain syndromes characterized by mechanical hypersensitivity.
Risk Factors & Causes
Early Life Stress Alters Brain Responses to Pain in Rats
Irritable bowel syndrome (IBS) is a disease estimated to affect approximately 12% of Americans. IBS is characterized by repeated stomach pains and possibly changes to bowel movements and is thought to be a problem of the interaction between the gut and the brain. Previous research has found that IBS patients are more likely to have experienced abuse, trauma or stress, particularly early-life stress as compared to healthy subjects. These findings led the authors of this paper to investigate whether stressors experienced in early life cause long term changes in brain properties and sensitivity (particularly in the abdominal region). To assess this hypothesis, the authors used a model of early life stress whereby bedding in the cage of newborn rats was restricted causing increased stress to the mother and decreased nurturing to the pups. The authors then tested to see whether the pups that were exposed to early life stress had higher sensitivity to a painful stimulus applied to the colon later in life and if this outcome was accompanied by changes in functional brain responses. Additionally, the authors assessed if there were differences, with respect to sensitivity and brain function, between male and female rats, which is important as females are more likely to suffer from IBS than males. What the authors found was that both male and female rats that were exposed to early life stress had higher sensitivity to a painful stimulus which was applied to the colon. The authors then went on to examine whether early life stress caused changes in brain function. They found that early life stress altered the response to a painful colonic stimulus in a number of brain circuits involved in pain and emotion. Moreover, connectivity of brain structures involved in pain and emotion were also altered by early life stress. Finally, when the authors assessed differences between male and female rats they found that though there was no difference in abdominal sensitivity between males and females, there were significant differences in the changes that early life stress caused in the brain of these rats. By identifying alterations induced by early life stress on brain responses to a painful colonic stimulus, this work has furthered our understanding as to how stress early on in life can impact abdominal pain, and potentially IBS later in life. Furthermore, this work highlighted differences in brain adaptations to early life stress, between males and females, which may help to better understand why females are more likely to be affected by IBS than males and may provide information for designing sex-specific targeted interventions to IBS.
Patients in"Pain Sensitive" Cluster are More Likely to Have Multiple Pain Disorders
Pain disorders are often classified based on the part of the body that is affected (e.g. back pain, jaw pain, headache). The fact that only the part of the body that is affected by pain, and not the underlying cause or biology of the pain, is considered when classifying pain disorders contributes in making pain hard to treat and manage. The authors of this report pointed out that risk factors for certain pain disorders have previously been identified and these risk factors indicate multiple different causes for the same type of pain. Therefore, the authors set out to classify subsets of chronic pain patients based on their risk factor profiles, hypothesizing that not all pain within a region of the body is the same. To carry this out the authors used 2 patient populations of temporomandibular disorder (TMD - pain classified as being in the jaw region) patients. The authors then identified factors related to pain sensitivity and psychological distress that were associated most with TMD and created 3 clusters of patients based on these factors. These clusters were named as 1) the adaptive cluster (higher pain thresholds, lower psychological distress) 2) the pain sensitive cluster (lower pain thresholds and lower psychological distress) and 3) the global symptoms cluster (higher pain sensitivity, higher psychological distress). The authors found that patients classified into the pain sensitive or global symptoms clusters reported higher clinical pain and higher frequency of comorbid pain symptoms than those clustered into the adaptive group. Interestingly, the authors then investigated whether these pain clusters had different incidences of pain disorders other than TMD. What the authors found was that patients clustered to the pain sensitive group had higher odds of having other pain disorders, when compared to the adaptive group, and the global symptoms group had higher odds of pain disorders than each of the other two groups. The authors then used these clusters to predict if subjects who did not have TMD would develop it and found that being categorized into the global symptoms cluster was predictive of the development of TMD. Therefore, using this methodology the authors were able to identify a subset of the population at risk for developing chronic pain, which is a tool public health professionals are lacking. Additionally, differences between these clusters provide evidence that all TMD does not have the same underlying biology, opening the door for more precise treatments for the various clusters of TMD.
Surveillance & Human Traits
Mindfulness-Based Stress Reduction May Help Sufferers of Low Back Pain

At any one-time, low back pain has been estimated to affect 540 million people across the world, making it the leading cause of disability globally. In fact, the burden of this symptom is so large that the prominent medical journal The Lancet recently published a series specific to low back pain that included a call to action. While surgical and pharmaceutical interventions are often used to treat low back pain, many guidelines call for non-pharmacological, psychological therapies to be used. In fact, cognitive behavioral therapy (CBT) has been demonstrated to be effective in treating low back pain, however, patients often have limited access to such interventions. In the current repot, authors aimed to test the effect of Mindfulness-Based Stress Reduction (MBSR) – a psychosocial approach that “focuses on increasing awareness and acceptance of moment-to-moment experiences” using a combination of meditation, body awareness and yoga – on low back pain (functional limitation and bothersomeness) in comparison to CBT or usual care. To carry this out, investigators recruited male and female participants, aged 20-70 years who had been suffering from non-specific low back pain for at least 3 months. Participants were then randomized to receive either usual care, or standardized CBT or MBSR interventions for 8 weeks and followed up for up to one year. The authors found that participants randomized to receive MBSR or CBT had reductions in pain-related functional limitations and pain bothersomeness that were significantly different from those randomized to receive usual care at 6 months, with effects of MBSR still present up to a year post randomization. Additionally, while both the MBSR and CBT groups did not differ in any pain related outcomes, CBT was more effective at improving depression at 8 weeks (i.e. at the end of the treatment phase). This was the first randomized clinical trial, with a large sample size, that assessed the effects of MBSR on chronic low back pain in a population not restricted to older adults. This paper demonstrates that both MBSR and CBT cause greater, clinically meaningful reductions in functional limitations and pain bothersomeness in patients suffering from chronic low back pain. The finding that MBSR is at least as effective as CBT for pain related improvements highlights the potential usefulness of MBSR as another psychosocial treatment that can be utilized by the many sufferers of chronic low back pain.
Tools/Instruments
New Imaging Technique Shows Connection Between Pain-Sensing Neurons
Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain.
Pain signals are mediated by neurons that stretch from the periphery back to the spinal cord, which group together to form specialized structures known as dorsal root ganglia (DRG). It has long been known that DRG neurons are involved in the act of nociception—the nervous system’s response to potentially harmful stimuli, but the ability to look at DRG neurons as a population has been limited. Researchers from Johns Hopkins University pioneered a way of imaging hundreds of DRG neurons together in live animals to study the effects of touch and nociceptive stimuli on these groups of neurons. By imaging a population of DRG neurons, the researchers were able to determine more about the neuronal crosstalk in response to a mechanical stimulus. As expected, the scientists found that injured mice—in models of either inflammatory or neuropathic pain—had an increased sensitivity to application of a mechanical force indicated by paw withdrawal. They then used genetically encoded calcium indicators to measure neuronal activity after application of the mechanical stimulus, and found that both kinds of injured mice had a higher proportion of neurons that fired together—which the scientists termed “coupled activation.” With increasing pressure from the mechanical stimulus, there was also an increasing proportion of coupled activation, but only in injured mice, suggesting that this is directly linked to hypersensitivity to the stimulus. To better determine what the explanation was for this neuronal crosstalk, the scientists used dyes injected into individual neurons, and observed that the dyes could be released into neighboring cells. This dye spreading implicates that the connection between DRG neurons is mediated by structures called gap junctions—molecular gates that directly link the cytoplasm of two cells allowing easy exchange of cellular material and markers such as dyes. When the scientists treated the injured mice with a drug that blocked the gap junctions, they did not observe the stimulus hypersensitivity that had been present without the gap junction blocker. The importance of this work is two-fold. First, the utility of imaging a large number of DRG neurons as a population will allow new questions about pain and neuronal crosstalk to be answered. Secondly, the implication of coupled activation via gap junctions could lead to new therapies that inhibit this cross talk and thus decrease hypersensitivity in individuals with pain.
