Download IPRCC Federal Pain Research Portfolio Analysis Report(pdf, 1056 KB)
Prepared by the Office of Pain Policy NINDS Linda Porter, Ph.D. Pain Policy Advisor Cheryse Sankar, Ph.D. Pain Policy Analyst Tara Schwetz, Ph.D. Pain Policy Analyst
The IPRCC and the Federal Pain Research Portfolio Analysis
The Patient Protection and Affordable Care Act (PPACA) includes a number of provisions designed to advance pain research, care, and education, including the creation of the Interagency Pain Research Coordinating Committee (IPRCC) by the Department of Health and Human Services (HHS). On behalf of HHS, the NIH established the IPRCC to coordinate all pain research efforts within HHS and across other Federal Agencies. The Committee is composed of seven Federal members and twelve non-Federal members, six drawn from the scientific and medical communities and six members of the public and stakeholder groups (a member roster is available at http://iprcc.nih.gov).
The Committee was given certain tasks to support the advancement of pain research that could best be addressed through a comprehensive analysis of the Federal pain research portfolio. These tasks include identifying critical gaps in basic and clinical research on the symptoms and causes of pain, and making recommendations to ensure that the activities of the National Institutes of Health and other Federal agencies are free of unnecessary duplication of effort.
Executive Summary
The Federal Pain Research Portfolio Analysis was undertaken to facilitate identification of gaps in the Federal pain research portfolio and to determine whether the National Institutes of Health and other Federal agencies are free of unnecessary duplication of effort. The Federal pain research portfolio has broad ranging interests, with areas of specialization within each agency based on their unique mission. The analysis revealed many areas of shared research interests between and across Federal entities, but no notable redundancies. Partnerships between Federal funding agencies have developed to leverage the research resources and data generated in some areas of shared interests. The broad, multidisciplinary nature of the portfolio reflects the complex mechanisms of chronic pain, the multifaceted risk factors for developing pain, and recognition of the need to individualize approaches to pain management through improved health care delivery. A detailed categorization of research projects identified synergy across agency interests as opportunity to collaborate in addressing specific unanswered questions.
Opportunities to advance the current research agenda emerged and gaps in the portfolio were identified by the analysis. It revealed needs for enhanced areas of research that are unique to specific chronic pain conditions and others that are relevant to all pain conditions. The research gaps that cut across pain conditions reflect promising areas to advance pain research, such as genotyping and genetic underpinnings of pain mechanisms and discovery of risk factors and mechanisms that underlie and predict the transition from acute to chronic pain. One promising area of basic research that focuses on central nervous system sensitization may help to determine the mechanisms for co-occurrence of multiple chronic pain conditions, but other avenues such as neuro-immune interactions, need to be explored to elucidate this phenomenon. Better tools for phenotyping and assessment that incorporate biopsychosocial components of pain for single and overlapping pain conditions are needed to enhance research and inform approaches to care. Translational research links basic research findings to the clinic. The development of more efficient treatment screening tools and approaches to care provides opportunities to bring needed novel pharmacological and non-pharmacological therapies into clinical evaluation. The importance of reverse translational research, applying clinical findings to direct basic research, is recognized, but remains an area in need of expansion. Clinical assessment tools for pain and outcome measures of pain management are not consistent across the research realm or in clinic practice. Development of consistent and meaningful instruments for individual patients and for population-level surveillance studies will generate quality data to further research and to inform care. Population level studies on quality of care and access to care, and the disparities that exist among different groups in obtaining appropriate pain management are of particular relevance with the changing health care environment.
Introduction
Numerous Federal agencies support pain research. To date, a comprehensive analysis of this broad and extensive research effort had not been undertaken. The NIH and the IPRCC developed and implemented the steps to complete a detailed analysis of pain research projects funded in fiscal year 2011. The reporting agencies included in this analysis are: the Agency for Healthcare Research and Quality (AHRQ), the Centers for Disease Control and Prevention (CDC), the Department of Defense (DoD), the Food and Drug Administration (FDA), the National Institutes of Health (NIH), and the Department of Veterans Affairs (VA), all of which have representation on the IPRCC (see Appendix for agency mission statements).
The portfolio analysis was performed in several stages by Federal staff, IPRCC members, and the NIH Office of Pain Policy. The process by which the 2011 data was collected, categorized, and evaluated will serve as the basis for future annual analyses of the portfolio. This report summarizes the findings and recommendations from multiple levels of analysis described below.
- At the inaugural IPRCC meeting, Federal members presented summaries of their high profile pain research programs and initiatives, and provided an overview of their portfolios. This effort indicated the breadth and complexity of the Federal portfolio, highlighted areas of shared interests and successful partnerships, and noted opportunities for synergy and available resources.
- The next step was to develop and implement a plan for a more detailed analysis of the portfolio. A working group of the IPRCC and NIH staff defined primary and secondary tiers of scientifically relevant topic areas into which more than 1200 federally funded research projects were categorized. The Tier 1 categories are broad: basic, translational, and clinical research. The Tier 2 categories define 29 narrow topic areas that are uniquely relevant to pain and meaningful to the broad range of agency missions and to the needs recognized by the pain research community (see Appendix for Tier 1 and 2 definitions). Staff across the Federal agencies coded their projects according to these primary and secondary categories. The Tier 2 category with the largest number of projects, Neurobiological Mechanisms of Pain, was divided into further detailed categories by IPRCC members. The specificity of the Tier 2 categories allows for a granular view of funded research in these topic areas.
- The narrowly defined Tier 2 categories then were organized into nine overarching research themes to present a broader view of cohesive units of related projects. These more global themes were compared to one another through an overlap analysis of projects that spanned multiple themes to reveal the level of cross-cutting, interdisciplinary research in the portfolio.
- As an additional layer of analysis, projects were coded by agency staff as relevant to a specific pain condition or a related group of conditions. IPRCC members, in collaboration with NIH staff, then selected subsets of projects related to a specific individual or set of grouped pain conditions. They analyzed the data from these subsets in depth to begin to identify unique and shared gaps across pain conditions, cross cutting areas in which enhanced collaborations would be beneficial, and research on underlying mechanisms that are relevant to multiple pain conditions.
- These multiple levels of analysis allowed a more in depth evaluation of the portfolio and a way to begin to identify gap areas and opportunities for future research. The NIH Office of Pain Policy summarized the data, outcomes, and recommendations from all stages of the analysis.
- This process for data collection, coding, and presentation was adapted for the development of an annually updated, automated, searchable database for the Federal pain research portfolio.
Portfolio Analysis – Overview
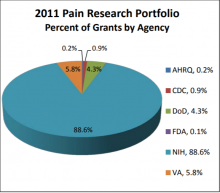
The 2011 Federal Pain Research portfolio includes more than 1200 grants and projects across the six reporting agencies. The NIH, whose primary mission is research, funds approximately 90% of these grants. Other agencies, whose primary missions address research as well as other aspects of public health safety and security, also provide an important contribution to the pain research effort. The DoD and VA fund approximately 4% and 5%, respectively, and AHRQ, CDC, and FDA each fund up to 1% of pain research (Figure 1).
Tier 1 and Tier 2 Analyses
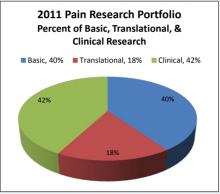
All grants were first coded as basic, translational, and/or clinical research (Tier 1 categories). Because many grants span more than one of these areas, a single grant may have been captured in more than one Tier 1 category. In that case, the project was assigned in designated proportions, not exceeding 100%, across Tier 1 categories. For example, if a project is 50% basic research and 50% translational research, it is assigned at 50% to each of the basic and translational Tier 1 codes. Overall, the 2011 grant portfolio comprises predominately basic (40%) and clinical (42%) grants with a relatively smaller proportion (18%) focused on translational research (Figure 2).
All grants then were coded according to scientific topic areas defined by the 29 Tier 2 categories. Many grants were relevant to more than one Tier 2 category, reflecting the multidisciplinary nature of the research. As with Tier 1 coding, an individual grant could be distributed in portions (not to exceed 100%) in multiple Tier 2 categories. Table 1 shows the percentage of grants across the 29 secondary categories. Coding for Tier 2 categories provides a detailed view of the distribution of grants across topic areas that are uniquely relevant to pain research. Because a large portion (20.4%) of the portfolio is devoted to basic research on neurobiological and glial mechanisms of nociception, a more detailed analysis of this category was performed by IPRCC members and is included in the Appendix. Coding each grant for both Tier 1 and Tier 2 categories served as a means to determine how topic areas relate to basic, translational, and clinical research.
Basic research includes understanding mechanisms that underlie nociception and pain perception; the role of genetic variants in altered pain and analgesic responses; mechanisms of therapies; neural circuitry of pain signaling; and altered brain function from chronic pain and its treatment. Much of the basic research is not condition specific, but relevant to all pain disorders.
Translational research spans numerous Tier 2 categories and includes targeted analgesic development, and assay and model development for basic and clinical research.
Clinical research includes development of instruments and resources for clinical research and pain care; interventional trials; population level studies on disparities in access to care and health care utilization; epidemiology of symptoms; disability; disease; susceptibility for chronic pain; addiction; and response to analgesics.
Research on education and training for various levels of career development is represented in many thematic areas and across the Tier 1 categories. Approximately 7% of all grants in the portfolio include a training component.
| Tier 2 Categories | % |
|---|---|
| Neurobiological/Glial Mechanisms | 20.4% |
| Pharm Mechanisms & Treatment | 8.3% |
| Non-Pharm Mechanisms & Treatment | 7.3% |
| Training in Pain Research | 6.9% |
| Biobehavioral & Psychosocial Mechanisms | 5.9% |
| Development of Animal & Human Pain Models | 5.2% |
| Outcomes & Health IT for Decision-making | 4.6% |
| Genetics & Genomics | 4.2% |
| Unique Populations | 4.2% |
| Mechanisms of Transition Phases | 4.1% |
| Pain & Non-Pain Comorbidities | 2.8% |
| Analgesic Development | 2.7% |
| Device & Therapy Delivery Systems Development | 2.3% |
| Comparative Effectiveness Research | 2.0% |
| Diagnosis & Case definitions | 1.9% |
| Epidemiology | 1.8% |
| Pain Education | 1.8% |
| Substance Use & Abuse/Addiction | 1.7% |
| Medical Management | 1.4% |
| Pain Prevention | 1.4% |
| Other “Omics” of Pain | 1.3% |
| Women’s & Minority’s Health Research | 1.3% |
| Informatics, Databases, & IT Development | 1.2% |
| Chronic Overlapping Conditions | 1.2% |
| Sex & Gender Differences | 1.1% |
| Analgesic Drug Safety | 0.9% |
| Pain and Trauma | 0.9% |
| Health Disparities & Access to Care | 0.9% |
| Health Care Utilization | 0.3% |
Analysis by Overarching Themes
The Tier 2 categories were organized into 9 overarching research themes to present cohesive units of related projects: Pain Mechanisms, Basic to Clinical, Disparities, Training & Education, Tools & Instruments, Risk Factors & Causes, Surveillance & Human Trials, Overlapping Conditions, and Use of Services, Treatments, & Interventions. Figure 3 shows how the Tier 2 categories were combined to form the themes.
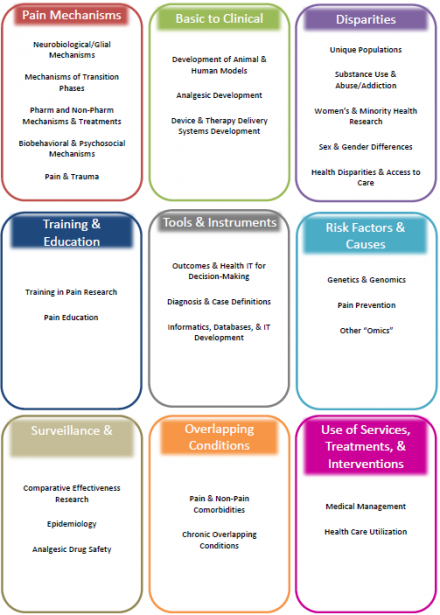
The distribution of grants across the nine research themes is shown in Figure 4. A more detailed description of the scope of projects within each theme is provided in the next section.
The thematic distribution of grants across agencies (Figure 5) reflects the mission-relevant research interests of each agency.
Pain Research Grants by Overarching Themes
Pain Mechanisms
Theme Definition: Biopsychosocial mechanisms underlying nociception, acute pain, the transition to chronic pain, chronic pain, analgesic targets, and treatment response. This theme includes the following Tier 2 categories: Neurobiological/Glial Mechanisms; Mechanisms of Transition Phases; Pharmacological Mechanisms & Treatments; Non-Pharmacological Mechanisms & Treatments; Biobehavioral & Psychosocial Mechanisms; and Pain & Trauma.
Pain Mechanisms is the largest overarching thematic group and comprises 42% of all grants. Half of this theme is composed of basic research grants (50%), which, for the most part, are not pain condition specific. Clinical (35%) and translational (15%) research grants account for the remaining half and often are focused on a specific pain condition (Figure 6A).
Overall, the grants in this theme span a very broad set of research topics. Their distribution across Tier 2 categories is shown in Figure 6B. They include studies on cellular and molecular mechanisms of nociception, how genetic variants affect susceptibility to chronic pain and analgesic response, and the transition to and maintenance of chronic pain, including altered peripheral and central nervous system function. This theme also includes research on biopsychosocial mechanisms that influence pain perception and treatment response, how treatment effects are manifested, and pain-condition specific etiologies.
All coded pain conditions are represented to some extent in this theme, with varying numbers of grants focused on different pain conditions. For example, 8% of grants primarily address osteoarthritis, 5% address each of cancer pain, neuropathic pain, LBP, and IBS. All other pain conditions are represented less frequently.
Forty-four percent of grants within this theme are coded in part in another theme as well (Figure 6C), which is defined here as overlapping with other overarching themes. These overlaps highlight the relationship between thematic areas of research and the multidisciplinary nature of grants in the portfolio. The Pain Mechanisms theme overlaps to the greatest extent with the Training & Education and Basic to Clinical themes. The high overlap between Pain Mechanisms and Training & Education is attributed to the large proportion of basic research training grants.
| Pain Mechanisms Theme Overlap | |
|---|---|
| Grants with overlap/no overlap | 44%/56% |
| Training & Education | 8% |
| Basic to Clinical | 7% |
| Risk Factors & Causes | 6% |
| Disparities | 4% |
| Tools & Instruments | 3% |
| Overlapping Conditions | 2% |
| Others | ≤ 1% |
Basic to Clinical
Theme Definition: Preclinical research that facilitates translation of basic research discoveries into clinically significant research tools and therapies. This theme includes the following Tier 2 categories: Development of Animal & Human Models; Analgesic Development; and Device & Therapy Delivery Systems Development.
Basic to Clinical grants constitute 11% of all Federally supported pain research, with the largest proportion representing basic research (45%), followed by translational research (34%), and then clinical research (21%) (Figure 7A). More than half of the grants in this theme are designed to develop and validate research tools, primarily condition, symptom, or mechanism based models that serve to advance a broad range of pain research. The remainder focuses on discovery, development, and testing of devices, therapy delivery systems, and novel analgesics to improve pain management. Their distribution across the Tier 2 categories is shown in Figure 7B.
A large proportion of grants in the Basic to Clinical theme are relevant to nociception or pain in general and are not focused on a specific pain condition. Of those that are condition specific, neuropathic pain grants are the most frequently represented of the conditions (9%), with headache, IC, IBS, and TMJD each representing 5% of the total. Other conditions are smaller portions of the total.
More than half (60%) of the grants in the Basic to Clinical theme overlap with other themes. Of those that overlap, 28% are coded for Pain Mechanisms, whereas other themes have only small percentages of overlap. The high level of overlap between Basic to Clinical and Pain Mechanisms themes is attributed to the importance of animal models to basic research.
| Basic to Clinical Theme Overlap | |
|---|---|
| Grants with overlap/no overlap | 60%/40% |
| Pain Mechanisms | 28% |
|
Pain Mechanisms, Risk Factors & Causes Training & Education |
5% |
| Pain Mechanisms, Training & Education | 4% |
|
Pain Mechanisms, Disparities Disparities |
3% |
|
Risk Factors & Causes Tools & Instruments Tools & Instruments Surveillance & Human Trials |
2% |
| Others | ≤1% |
Disparities
Theme Definition: Research on differences in susceptibility to pain and the pain experience among unique and diverse populations, including racial and ethnic groups, the elderly, children, military personnel, people with disabilities, populations with abuse/addictive behaviors, gender groups and end of life patients. The theme also includes research on disparities to access and in quality of pain management. This theme includes the following Tier 2 categories: Unique Populations; Substance Use & Abuse/Addiction; Women’s & Minority Health Research; Sex & Gender Differences; and Health Disparities & Access to Care.
The Disparities theme comprises 10% of all grants. The large majority of grants within this theme are classified as clinical (74%), with only 15% basic and 12% translational research (Figure 8A). This breakdown reflects the large number of clinical intervention and outcome studies that target a number of subgroups including sex and gender and age specific populations. Translational research grants have a strong focus on the elderly and on abuse and addiction.
Of the Tier 2 categories within this theme, Unique Populations contains the largest number of grants, as it encompasses several different groups (Pediatric, Elderly, End of Life, Disabled, and Military). The predominance of chronic pain conditions in women may account for the large number of grants in the Tier 2 category Sex and Gender Differences, many of which are basic research grants that target sex differences and women’s health.
A large portion (18%) of grants within this theme is not specific to any pain condition. Of those grants that are pain condition specific, 12% are focused on cancer pain, 7% on osteoarthritis, 6% on IBS, and 5% to each of headache, sickle cell pain and low back pain, with other conditions having a smaller percent.
More than two thirds of grants in the Disparities theme overlap with other themes. The Disparities theme overlaps primarily with Pain Mechanisms, likely because of the emphasis of basic science research grants on sex differences.
| Disparities Theme Overlap | |
| Grants with overlap/no overlap | 79%/21% |
| Pain Mechanisms | 19% |
| Training & Education | 10% |
| Tools & Instruments | 6% |
| Pain Mechanisms, Tools & Instruments | 5% |
|
Surveillance & Human Trials Use of Services, Treatments & Interventions |
4% |
|
Basic to Clinical Overlapping Conditions Basic to Clinical, Pain Mechanisms Pain Mechanisms, Training & Education |
3% |
|
Risk Factors & Causes Risk Factors & Causes, Overlapping Conditions Surveillance & Human Trials, Use of Services, Treatments, & Interventions |
2% |
| Others | 1% |
Training & Education
Theme Definition: Basic and clinical research training for scientists at various career stages and education related to pain for health care providers, caregivers, and patients. This theme includes the following Tier 2 categories: Training in Pain Research and Pain Education.
The Training & Education theme comprises 10% of the total Federal pain research portfolio. Nearly half (47%) of the Training & Education grants are clinical research projects; 35% are basic and 18% are translational (Figure 9A).
Most grants within this theme are categorized as Tier 2, Training in Pain Research (80%). The NIH and the VA use these grants to support mentored pain research training for basic and clinical investigators with project topics ranging across the spectrum of the Federal pain research portfolio. Grants categorized as Tier 2, Pain Education, support research to develop, improve, and evaluate educational tools and resources, primarily for health care providers and caregivers. There also are a small percentage of grants in this theme that provide educational programs for patients based on evidence obtained through pain research.
The majority of grants within Training & Education are not focused on a specific pain condition. Of those grants that are condition specific, cancer pain accounts for 9% of grants, headache for 6%, and IBS, osteoarthritis, rheumatoid arthritis, and sickle cell pain each account for 3%. Other conditions are represented to a lesser extent.
The Training & Education theme overlaps extensively (85%) with other themes, mainly because the mentored training grants cover broad research topics. Only 15% of grants are coded only for the Training & Education theme. There is significant overlap with the Pain Mechanisms (35%) and Disparities themes (10%), and some level of overlap with all other themes (Figure 9C).
| Training & Education Theme Overlap | |
| Grants with overlap/no overlap | 85%/15% |
| Pain Mechanisms | 35% |
| Disparities | 10% |
|
Basic to Clinical Risk Factors & Causes |
5% |
| Basic to Clinical, Pain Mechanisms | 4% |
|
Pain Mechanisms, Risk Factors & Causes Pain Mechanisms , Disparities Tools & Instruments Overlapping Conditions |
3% |
|
Surveillance & Human Trials Use of Services, Treatments, & Interventions Pain Mechanisms, Tools & Instruments |
2% |
| Others | ≤1% |
Tools & Instruments
Theme Definition: Development and validation of clinical research tools and infrastructure including data bases and registries. This theme includes the following Tier 2 categories: Outcomes & Health IT for Decision-Making; Diagnosis & Case Definitions; and Informatics, Databases, & IT Development. Tools & Instruments grants comprise 8% of the Federally-supported pain research portfolio. Grants in this theme focus primarily on tools and infrastructure for clinical research, with only a small percent that support translational (15%) and basic (9%) research endeavors (Figure 10A).
The projects include research to improve clinical study design and to support clinical studies through development of diagnostic and assessment instruments, such as novel pain self-reports, and physical, cognitive, emotional, and disability measures.
Other studies in this theme enhance the clinical research endeavor by establishment of networks, consortia, and collaborations for harmonization of data collection and analysis. Information technologies are applied to enhance data extraction (e.g. from EMR) and comparability of pain data sets (e.g. ontology for pain). There also is a set of grants to improve methodology and technology for clinical research and patient outcomes. The distribution of grants into the Tier 2 categories is shown in Figure 10B. The theme is of particular relevance to population research.
Even though most of the research in this theme is clinical, 18% of the grants do not focus on a specific pain condition. Osteoarthritis is the most highly represented pain condition in this theme (19%), followed by low back pain (10%), sickle cell pain (5%), and rheumatic pain (5%). Other pain conditions are represented to a lesser extent.
More than half (60%) of the grants in Tools & Instruments overlaps with other themes. The most overlap is with the Pain Mechanisms theme (16%) and to a lesser extent (7%) with the Disparities theme (Figure 10C), indicating the research resources targeting these areas of research.
| Tools & Instruments Theme Overlap | |
|---|---|
| Grants with overlap/no overlap | 60%/40% |
| Pain Mechanisms | 16% |
| Disparities | 7% |
| Pain Mechanisms, Disparities | 6% |
| Surveillance & Human Trials, Training & Education | 4% |
| Risk Factors & Causes | 3% |
|
Basic to Clinical Basic to Clinical, Surveillance & Human Trials Pain Mechanisms, Risk Factors & Causes Pain Mechanisms, Surveillance & Human Trials Pain Mechanisms, Training & Education |
2% |
| Others | ≤1% |
Risk Factors & Causes
Theme Definition: Research on the biopsychosocial risk factors, susceptibility, and signatures for persistent pain, and development and testing of methods, interventions, and approaches to prevent chronic pain. This theme includes the following Tier 2 categories: Genetics and Genomics; Pain Prevention; and Other “Omics.”
The Risk Factors & Causes theme comprises 8% of all grants in the Federal portfolio. More than half of the grants in this theme are categorized as basic (45%) and translational (20%) research with the remainder as clinical (35%) (Figure 11A). The basic and translational projects include research to identify genetic variants associated with specific chronic pain conditions or chronic pain (non-condition specific). Studies seek to determine the role of “pain genes” in underlying mechanisms or as modifiers of pain and, as such, how they contribute as risk factors for chronic pain. Genetic approaches are used in translational studies to determine new therapeutic targets and to develop novel therapies. The current interest on genes in research is reflected in the high percent (61%) of grants in this theme from the Tier 2 category Genetics and Genomics (61%). Other biopsychosocial mechanisms and risk factors also are represented in this theme. The Tier 2 category of pain prevention accounts for 20% of the grants in this theme. These grants target pain prevention through various approaches including self-directed activity, diet, life style programs and education campaigns for many disorders, notably low back pain and osteoarthritis. In addition, both basic and clinical studies on prevention seek to develop and test preemptive approaches to prevent pain resulting from treatments or procedures, and to facilitate symptom management of non-pain contributors to pain, such as stress. Other studies examine disease progression and aim to develop preventive therapies for episodic pain conditions.
Over a quarter of the grants in this theme are not associated with a particular pain condition, and are primarily basic research projects that focus on mechanisms of nociception or non-specified pain conditions. Of the grants that focus on a specific pain condition, 8% are relevant to IBS, 18% to neuropathic pain, 14% to osteoarthritis and rheumatoid arthritis, and 4% to TMJ. Other conditions are represented in this theme but to a lesser extent.
A large portion (77%) of these grants overlap with other themes, especially with the Pain Mechanisms theme (36%).
| Risk Factors & Causes Theme Overlap | |
|---|---|
| Grants with overlap/no overlap | 77%/23% |
| Pain Mechanisms | 36% |
| Basic to Clinical, Pain Mechanisms | 7% |
| Training & Education | 6% |
| Pain Mechanisms, Training & Education | 4% |
|
Basic to Clinical Pain Mechanisms, Overlapping Conditions Tools & Instruments |
3% 3% 3% |
|
Pain Mechanisms, Tools & Instruments Disparities Disparities, Overlapping Conditions |
2% 2% 2% |
| Others | ≤1% |
Surveillance & Human Trials
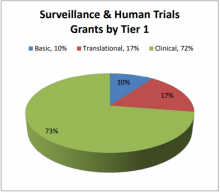
Theme Definition: Population research on the prevalence and incidence of pain and the burden of pain across the disease progression and recovery and through the lifespan. This theme also includes clinical studies of research methods and interventions, models of care delivery systems and providers, and safety and use of pain medications. This theme includes the following Tier 2 categories: Comparative Effectiveness Research, Epidemiology, and Analgesic Drug Safety.
The Surveillance & Human Trials theme comprises 5% of the Federal portfolio, with grants funded by all six reporting agencies. Given the patientfocused nature of this theme, most of the grants (72%) are categorized as clinical research, with a relatively small portion of basic (10%) and translational (17%) research (Figure 12A).
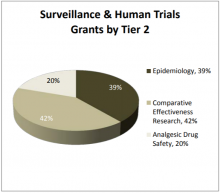
Of the Tier 2 categories, 42% of grants are categorized as Comparative Effectiveness Research. The AHRQ, DoD, NIH, and VA fund comparative effectiveness studies on a wide range of interventions and approaches to care. The DoD in particular focuses on comparative effectiveness of complementary and alternative modalities (CAM). Pursuant to their mission, the FDA supports grants classified as Tier 2 Analgesic Drug Safety. The DoD, NIH, and VA also support trials from the Analgesic and Drug Safety category, primarily on safety and toxicity of drugs under development and adverse effects of drugs (especially opioids) currently in use. Epidemiologic studies also cover a broad range of pain conditions, as well as risk factors, disease burden, and disease progression.
A large portion of grants within Surveillance & Human Trials are not specific to a particular pain condition. As reflective of the large populations covered in this theme, they include studies that address chronic pain that is not condition specific in groups, such as the elderly, studies on sleep and pain, emotional regulation and pain, and symptom (pain) management. Of those coded for this theme, LBP (10%), trauma pain (5%), headache (5%), IBS (4%), pelvic pain (4%), CFS (3%), TMJ (3%), phantom limb pain (3%), and neuropathic pain (3%) account for most, but not all conditions.
Surveillance & Human Trials grants overlap significantly with many, but not any particular theme to a great extent.
| Surveillance & Human Trials Theme Overlap | |
| Grants with overlap/no overlap | 72%/28% |
|
Pain Mechanisms Disparities |
7% |
| Tools & Instruments | 6% |
|
Overlapping Conditions Training & Education |
4% |
|
Basic to Clinical, Tools & Instruments Pain Mechanisms, Tools & Instruments Pain Mechanisms, Overlapping Conditions Use of Services Treatments & Interventions, Disparities |
3% |
|
Basic to Clinical Basic to Clinical, Pain Mechanisms Pain Mechanisms, Use of Services, Treatment, & Interventions Pain Mechanisms, Disparities Pain Mechanisms, Training & Education Risk Factors & Causes, Disparities Tools & Instruments, Disparities Use of Services, Treatments & Interventions |
2% |
| Others | 1% |
Overlapping Conditions
Theme Definition: Research on biopsychosocial mechanisms shared across overlapping pain conditions and pain and non-pain conditions, as well as relevant treatment approaches and population studies. This theme includes the following Tier 2 categories: Pain & Non-Pain Comorbidities and Chronic Overlapping Pain Conditions.
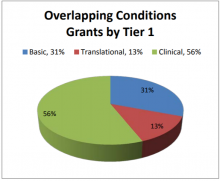
The Overlapping Conditions theme comprises 4% of all grants. The majority of grants in this theme are patient focused and support clinical studies (56%). Basic (31%) and translational (13%) research portions are relatively smaller (Figure 13A).
Nearly 70% of grants in this theme are in the Pain & Non-Pain Comorbidities Tier 2 category (Figure 13B). These studies cover a broad range of conditions that frequently are associated with chronic pain, including anxiety, depression, stress, PTSD, and sleep disturbances.
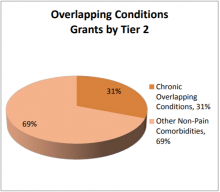
The remaining grants in this theme (31%) cover mechanisms, assessment, research, and treatment approaches for multiple pain conditions in an individual.
Most of the grants in the portion of this theme that relate to overlapping pain conditions are not specific to any single pain condition, but to various combinations of conditions. Headache (10%), trauma pain (6%), pelvic pain (6%), TMJ (4%), FM (2%), IC (2%), endometriosis (2%), and orofacial (2%) pain are among the pain conditions most represented, which correlates with the clinical prevalence of these conditions as co-existing pain conditions.
Two thirds (68%) of the Overlapping Conditions grants overlap with other overarching themes, including primarily Pain Mechanisms (20%), Disparities, and Training & Education.
| Overlapping Conditions Theme Overlap | |
|---|---|
| Grants with overlap/no overlap | 68%/32% |
| Pain Mechanisms |
20% |
| Disparities | 7% |
| Training & Education | 6% |
|
Pain Mechanisms, Risk Factors & Causes Surveillance & Human Trials |
5% |
|
Pain Mechanisms, Surveillance & Human Trials Risk Factors & Causes, Disparities |
4% |
|
Basic to Clinical, Pain Mechanisms Pain Mechanisms, Training & Education |
2% |
| Others | 1% |
Use of Services, Treatments, & Interventions
Theme Definition: Research on integrated team based and self-management approaches to pain, as well as use and barriers to health care, and transitions within health care settings. This theme includes the following Tier 2 categories: Medical Management and Health Care Utilization.
The Use of Services, Treatments, & Interventions theme comprises 2% of grants in the Federal portfolio. The agencies that support research on this theme are the NIH and the VA. Given the relevance of the theme to pain management and patient care, the vast majority of grants are clinical (80%), and the remaining are translational (20%). There are no basic research grants in this theme (Figure 14A).
The majority of grants within this theme are in the Medical Management category (81%). Many of the studies assess models or approaches to care in different settings, while others aim to evaluate interventions in novel health care settings or to evaluate novel modes of care and self-care delivery. The Health Care Utilization research is focused on use of various treatments in different health care settings, adherence to treatment, and self-management strategies.
Pain conditions represented in this theme include LBP (17%), sickle cell pain (16%), and cancer pain (16%). Given the small number of grants in this theme, some other, but not all, pain conditions are represented. In addition, many of the grants (26%) are not specific to a particular pain condition.
Significant overlap exists (86%) with other overarching themes, with Disparities and Training & Education representing the highest percentage of overlap (20% and 11%, respectively).
| Use of Services, Treatments, & Interventions Theme Overlap | |
| Grants with overlap/no overlap | 76%/14% |
| Disparities | 20% |
| Training & Education | 11% |
| Surveillance & Human Trials, Disparities | 9% |
|
Pain Mechanisms Surveillance & Human Trials Pain Mechanisms, Disparities Pain Mechanisms, Surveillance & Human Trials |
6% |
| Others | 3% |
Pain Research Grants by Pain Conditions
A large percent of the grants in the portfolio were coded as relevant to a pain condition. Pain grants that are not focused on a particular condition (approximately 34% of the portfolio) are not included in this portion of analysis. The data presented here are for both single and grouped pain conditions. Because many pain conditions have similar symptomatology, affect related body parts, and may share mechanisms, they are defined here as grouped conditions. For example, peripheral neuropathies include painful diabetic neuropathy, HIV/AIDS painful neuropathy, post herpetic neuralgia, trigeminal neuralgia, treatment induced neuropathy, Charcot Marie Tooth, carpal tunnel syndrome and other entrapment neuropathies. The specific disorders included in each grouped condition are listed in the Appendix.
Figure 15 illustrates the distribution of Basic, Translational, and Clinical grants for grouped pain conditions. Research on most grouped conditions is primarily clinical, except for neuropathic pain, chronic pelvic pain and most notably TMJ/orofacial grants, which represent a large component of basic research grants.
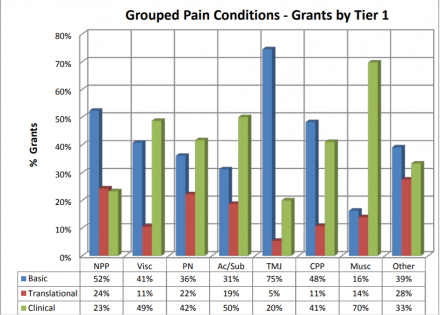
NPP: Neuropathic Pain; Visc: Visceral Pain; PN: Peripheral Neuropathies; Ac/Sub: Acute/SubAcute Pain; TMJ: Temporomandibular Joint Disorder and Orofacial Pain; CPP: Chronic Pelvic Pain; Musc: Musculoskeletal Pain; Other: Other Pain Conditions
Figure 16 illustrates the distribution of Basic, Translational, and Clinical grants for single pain conditions. Research studies are primarily clinical for all single conditions, except headache which has slightly more basic than clinical research grants.
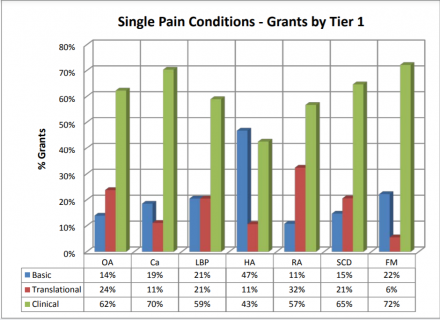
OA: Osteoarthritis; Ca: Cancer Pain; LBP: Low Back Pain; HA: Headache; RH: Rheumatoid Arthritis; SCD: Sickle Cell Disease; FM: Fibromyalgia
The heat map in Table 2 demonstrates the distribution of Tier 2 studies for the grouped pain conditions. Of the 29 categories, Neurobiological/Glial Mechanisms represents over 20% of the grants in 6 of the 8 conditions. Pharmacological Mechanisms & Treatments, Non-Pharmacological Mechanisms & Treatments, Training in Pain Research, Development of Animal & Human Models, and Biobehavioral and Psychosocial Mechanisms also accounted for a large proportion of grants within the grouped conditions.
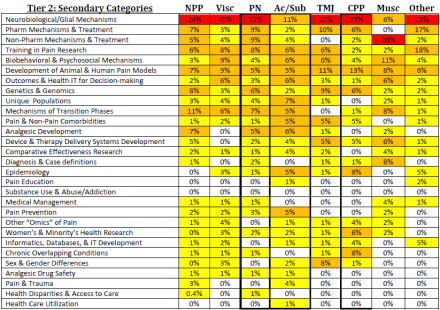
NPP: Neuropathic Pain; Visc: Visceral Pain; PN: Peripheral Neuropathies; Ac/Sub: Acute/SubAcute Pain; TMJ: Temporomandibular Joint Disorder and Orofacial Pain; CPP: Chronic Pelvic Pain; Musc: Musculoskeletal Pain; Other: Other Pain Conditions
The heat map in Table 3 demonstrates the distribution of Tier 2 studies for the single pain conditions. Of the 29 categories, Non-Pharmacological Mechanisms & Treatments is highly represented for many of the pain conditions. Outcomes & Health IT for Decision Making, Biobehavioral & Psychosocial Mechanisms, and Neurobiological/Glial Mechanisms also accounted for a large portion of grants for many conditions.
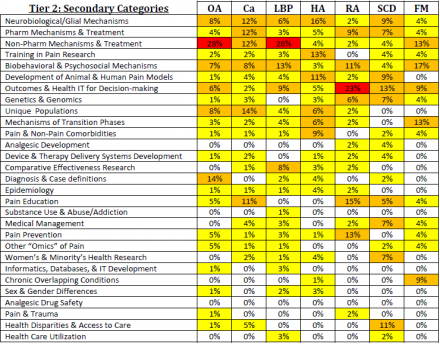
OA: Osteoarthritis; Ca: Cancer Pain; LBP: Low Back Pain; HA: Headache; RA: Rheumatoid Arthritis; SCD: Sickle Cell Disease; FM: Fibromyalgia
Identifying Opportunities for Synergy
An overview of the pain portfolios of all Federal Agencies represented on the IPRCC was developed as a first step of the portfolio analysis to determine highlights, potential redundancies, and collaborative opportunities in research efforts across the Federal Agencies that support pain research. This helped inform the more detailed analysis and also offered insights into shared interests across agencies.
Shared Interests in Basic Research
Underlying mechanisms of injury in acute and chronic pain as a consequence of trauma
- DoD - Mechanisms of pain caused by battlefield and training trauma, including burn, amputation, and inflammation
- NIH - Peripheral and central mechanisms of acute and chronic pain as a consequence of burn and nerve injury
- VA - Chronic pain as a consequence of trauma from burn and blast injuries
Shared Interests in Translational Research
Preclinical therapy development and testing for neuropathic pain as a consequence of trauma
- DoD - Develop opioid-alternative therapies for pain caused by traumatic battlefield and training injury
- NIH - Discovery, development, and preclinical testing of novel pharmacological and non-pharmacological interventions to assess therapeutic effectiveness
- VA - Preclinical testing and development of therapies for injury from trauma
Technology for optimizing analgesic delivery systems
- DoD - Analgesic delivery systems for pain from traumatic injury
- NIH - Optimization of therapeutic response through improved drug delivery for acute and chronic pain
Shared Interests in Clinical and Comparative Effectiveness Research
Pharmacological and non-pharmacological treatment and management of lower back pain
- AHRQ - Behavioral interventions and acupuncture for acute and chronic low back pain
- DoD - Clinical trials to evaluate pharmacological and adjunct therapies, including integrative medicine, for low back pain
- NIH - Evaluation of analgesics and adjunct complementary and alternative medicine (CAM) interventions for management of low back pain
Treatment and management of pain in osteoarthritis
- AHRQ - Comparative effectiveness studies on osteoarthritis
- NIH - Development and evaluation of interventions to ameliorate pain in orthopedic disorders
Patient data registries for pain
- DoD - Pain registries for military personnel
- NIH - Tools and infrastructure for pain registries
- VA - Musculoskeletal diseases cohort
Predictors, risks, and management of prescription drug abuse and misuse
- DoD - Opioid use and detoxification
- NIH - Programs to prevent abuse/misuse of prescription analgesics (especially opioids) and to manage opioid use
- VA - Improving the quality and safety of opiate prescribing
Shared Interests in Epidemiology and Health Disparities Research
Trends in use and abuse of prescription and over-the-counter analgesics
- CDC - Study of chronic pain prevalence and treatment with prescription drugs
- FDA - Opioid use, abuse, and prescribing patterns
- NIH - The impact of analgesic treatment on pain and associated symptoms, and medication concerns, especially opioids, in the elderly
Prevalence and risk factors for arthritis and low back pain
- AHRQ - Prevalence of low back pain
- CDC - Surveillance of health practices and risk behaviors relevant to arthritis pain
- NIH - Racial, ethnic, and sex differences in pain and quality of care at the individual and community level, racial differences in osteoarthritis, and preferences in health care management for arthritis
Tracking long-term outcomes of chronic pain and co-morbid psychological conditions as a consequence of injury
- DoD - Tracking Post Traumatic Stress Disorder (PTSD) and amputation
- VA - Epidemiology of physical injuries and mental illness in veterans and treatment differences between men and women with PTSD, TBI, depression and pain
Shared Interests in Training, Education and Dissemination
Training for health care providers and researchers, and public education
- DoD - Health care provider training for pain management
- CDC - Physician training for arthritis pain referrals to effective interventions through academic details
- FDA - Training of opioid prescribers through academic details and evaluating prescribing habits
- NIH - Institutional training programs for research training in anesthesia; training for addiction medicine skills; career development programs for training in basic and clinical pain research
- VA - Mentored training in pain research
Summary and Conclusions on Opportunities for Synergy
Overall, the Federal pain research portfolio has broad ranging interests, with areas of specialization within each agency based on their unique mission. The portfolio overview revealed many areas of overlapping interests in the cross-agency portfolio. Detailed analyses indicate that the research within these overlapping topic areas is not redundant, but for the most part is complementary. In some instances, the agencies have developed ongoing collaborative efforts to facilitate overlapping areas of research interests. These are highlighted under “Ongoing Partnerships.” There remain, however, opportunities to enhance the Federal research effort through collaboration between and across agencies. This review highlights potential opportunities for further or future collaborative efforts among or between agencies.
In summary, the areas of greatest shared interests include:
- Peripheral and central nervous system mechanisms underlying chronic neuropathic pain as a consequence of trauma, and discovery and development of treatment strategies, including analgesic delivery systems
- Clinical research to develop and evaluate pharmacological, behavioral, and adjunct treatment programs and population level research to assess prevalence, pain management, health practices and risk behaviors, and access to care for low back pain and osteoarthritis
- Basic, translational, and clinical research to develop and evaluate alternatives to opioid therapy, and to manage opioid use and prevent misuse and abuse; population level research to identify prescribing patterns and risk factors, and predictors for opioid misuse and abuse; training programs for addiction medicine and safe opioid prescribing
Current Federal Research Partnerships for Pain
Pain Assessment Screening Tool and Outcomes Registry (PASTOR) and Patient Reported Outcomes Measurement Information System (PROMIS)
DoD and NIH
PASTOR/PROMIS provides a system to track standardized outcome data for pain management. This system is a combination of the NIH PROMIS and the DoD PASTOR initiatives. PROMIS is a set of computer-adaptive testing, reliable tools that provide patient-reported health status on psychosocial and physical functioning and includes a pain domain. The PASTOR system will collect clinical information from the patient about their pain and summarize the information for clinicians and medical resource managers. The DoD is developing the information technology requirements documents to establish PASTOR/PROMIS as an information system.
Research on Complementary Approaches to Symptom Management in Military and Veteran Populations: Notice of Funding Opportunity PA-13-075
NIH, DoD, and VA
This initiative is focused on complementary approaches to pain and symptom management or improving health among U.S. military personnel, veterans and their families. The goal of the announcement are to foster research collaborations between NCCAM-funded researchers and VA or DoD clinicians or researchers to conduct research on complementary, non-pharmacological, or integrative approaches to symptom management and health in military or VA populations.
Chronic Effects of Neurotrauma Consortium Award: Funding Opportunity W18XWH-12
DoD and VA
The goal of this initiative is to understand the chronic sequelae associated with neurotrauma, primarily TBI. The Consortium will coordinate research activities, serve as a data coordinating center and establish a specimen repository. One of the Consortium’s activities is development and coordination of research projects on comorbidities of neurotrauma, including chronic pain/headache.
Acupuncture Training Across Clinical Settings: Joint Incentive Fund Initiative Proposal
DoD and VA
The goal of this initiative is to develop, pilot, evaluate, and implement an acupuncture education and training program for DoD and VA health care providers across military and VHA treatment facilities. It will serve to integrate acupuncture into integrated care for pain management in military and VA medical facilities.
Pain Care Education and Training Curriculum: Joint Incentive Fund Initiative Proposal
DoD and VA
The goal of this initiative is to develop, evaluate, disseminate, and integrate a core pain management education and training curriculum that will contribute to the implementation of standardized, interdisciplinary, biopsychosocial and integrated stepped care pain management across DoD and VA health systems.
Highlighted Pain Research Analyses
The set of pain conditions included in these detailed analyses are representative samples from the federal pain research portfolio. Their selection was based primarily on the expertise of the IPRCC members who performed the analyses. In some cases, IPRCC members were assisted by NIH program staff with relevant expertise.
Sickle Cell Disease Pain: Wally Smith, IPRCC and Harvey Luksenburg, NHLBI
Sickle cell disease (SCD) is a rare autosomal recessive disease (100,000 in US), which begins as acute-on-chronic, multi-local vaso-occlusive, ischemic and inflammatory pain. The phenotype appears to transform over time to central and/or peripheral neuropathic pain.
Overview of the Portfolio
The largest percentage of grants focus on pain outcome assessments and measures (13%), health disparities in sickle cell pain, its management and access to care (11%), genetics and genomics of nociception and pain (7%), medical management of pain (7%), pharmacological mechanisms and treatment (7%), and development and validation of animal models. Compared with other conditions, SCD has a higher percentage of grants in pain outcome assessments, in health disparities, and pain and women’s and minority’s health research.
Gaps
- Understanding of SCD pain phenotype(s) or diagnosis/case definitions, especially neurological, inflammatory, biobehavioral, and psychosocial mechanisms
- Targeted development of analgesics, device, and therapy delivery systems to neurological, inflammatory, biobehavioral, and psychosocial mechanisms of pain
- Understanding whether the pain phenotype in SCD is static or age-dependent, and thus susceptible to different therapeutic approaches at different life-stages
- Determining how genetic or other mechanisms alter the SCD pain phenotype (represented, but under-researched)
- Recruitment of SCD Subjects (who often respond sub-optimally to opioids) into clinical trials of new, nonopioid therapeutics
- SCD pain in pediatric and other unique populations
- Pain education, omics of pain, and non-pharmacological treatment mechanisms
Commonalities with Other Pain Conditions
Chronic SCD pain, which is a major source of morbidity in adolescents and adults, has phenotypic features in common with conditions that exhibit features of central sensitization syndromes, such as Irritable Bowel Syndrome (IBS), chronic low back pain (LBP) and chronic headache.
Shared Interests and Opportunities to Collaborate
Subjects with SCD have complex pain patterns. Thus, pain phenotyping, especially for chronic SCD pain, is a priority for clinical research. Cooperation with the OPPERA (NIDCR) and MAPP (NIDDK) studies will be beneficial. Recently, NHLBI investigators have begun interdisciplinary collaboration with the NINDS, NIDCR, NCCAM, and NIDA to advance the research agenda on pain in SCD.
Cancer Pain: Christine Miaskowski, IPRCC and Ann O’Mara, NCI
Unrelieved pain occurs in 30-50% of oncology patients on active treatment, 30-40% of cancer survivors, and 80- 90% of terminally ill cancer patients. It is the most feared symptom associated with cancer and treatment. Cancer pain has unique characteristics and numerous etiologies including the disease itself and its treatment, and patients may experience multiple types of cancer pain, along with non-cancer pain. Underlying mechanisms for different types of pain may be unique and warrant different interventions.
Overview of the Portfolio
Overview of the Portfolio Preclinical descriptive studies (19%) focus on interactions between cancer cells and sensory neurons that result in algesia or inhibit analgesic responses. Models utilized include bone pain, pancreatic cancer, prostate cancer, and the stromal cell environment. Mechanistic targets are being investigated including chemokines, capsaicin, endocannabinoid signaling, and vanilloid receptors. Clinical descriptive studies (15%) focus on the biological basis for symptom clusters in oncology patients in which pain is one symptom. Inflammation and sickness behavior are being investigated as the basis for the symptom cluster that includes pain. Other studies focus on optimizing patient-reported outcomes. Preclinical intervention studies (8%) include development and testing of spinal gene therapy delivery, herbal treatment, cannabinoid CB2 agonists, and anti-NGF and TrkA blockers for treatment or prevention of cancer pain. Clinical intervention studies (45%) include cancer pain specific pharmacologic and non-pharmacologic treatment for multiple symptoms in patients undergoing treatment or in survivors (in part to decrease stress and inflammation) and assessment of treatment or palliative care when pain is a secondary outcome. Education studies (2%) are focused on palliative care education for care providers in adult, pediatric, and unique populations.
Gaps
- Development of appropriate and additional animal models for cancer pain and for common pain conditions associated with cancer and cancer treatment
- Development of mechanistically-based analgesics
- Phenotyping and genotyping of common cancer pain syndromes
- Evaluation of how persistent pain impacts survivors and patients’ treatment and survival
- Impact of transitions in care (from oncologist to primary care) on pain management
- Impact of cancer pain on special populations (e.g. pediatrics, elderly, ethnicity, survivors)
Commonalities with Other Pain Conditions
Preclinical behavioral models for cancer pain and pain mechanisms of cancer-sensory neuron interactions may prove useful for studying other pain conditions. Information on mechanisms that underlie and impact symptom clusters, including pain may apply to other chronic conditions with pain as a symptom.
Shared Interests and Opportunities to Collaborate
Potential shared interests across agencies or NIH Institutes include cancer-sensory neuron interactions, symptom clusters in oncology patients, and provision of palliative care services.
Migraine: Michael Moskowitz, IPRCC
Migraine is the most common neurological disorder, affecting 12% of the population. Recent discoveries offer opportunities to advance headache research.
Overview of the Portfolio
Major basic science themes focus on mechanisms of pain; ion channel function in meningeal afferents, peripheral and central CGRP receptor function, and central neuronal processing and circuitry. The role of genetic mutations and use of genetic models to study modulatory effects of neurotransmitters and hormonal influences are included. Other studies characterize the role of mast cells and explore how stress precipitates headache. Migraine with aura is the predominate headache subtype represented in the portfolio, with studies on mechanisms of pain initiation, sex hormone effects on cortical spreading depression (CSD) threshold, blood vessel regulation, stroke susceptibility, CSD screens of clinically relevant drugs, and computational models to explore physiological events underlying CSD. Development and validation of animal models for CSD, chronic daily and medication overuse headaches, and photophobia are emerging. Clinical studies include imaging using MRI to correlate CNS characteristics with migraine frequency and to investigate functional networks in migraine, PET to explore u-opioid receptor binding abnormalities relative to brain phenotype, and MEG to assess cortical changes in pediatric migraine. Interventional studies include a pediatric trial to determine first choice preventive medication and non-pharmacological interventions for cervicogenic and tension type headaches. Other clinical studies have a strong behavioral focus and explore pediatric migraine, and migraine related to cerebrovascular disease and stroke. More than 29% of the grants were specified for training as well as for research.
Gaps
- more coherence is needed for studies related to central mechanisms of migraine
- post-traumatic headaches (PTH), among the most difficult to treat, are underrepresented
- unmet need for biomarkers to aid diagnosis, prognosis, and treatment options
- mechanistic studies in chronic daily headache
- drug development for migraine prevention
Commonalities with Other Pain Conditions
- Migraine may transition from an episodic to a chronic pain condition
- Placebo response must be considered in design and interpretation of trials for chronic pain/headache
Shared Interests and Opportunities to Collaborate
Alliances are warranted between the field of headache and co-morbid conditions such as pain, epilepsy, sleep, obesity, depression, and anxiety. Investigation into integrated self-management strategies for chronic pain is of broad interest for pain and reflects collaborative opportunities. PTH research could be advanced by partnering with the military, sports medicine, and industry. Migraine genetics is underrepresented in the portfolio for typical migraine subtypes, but large consortia have been well established in Europe to address this topic.
Chronic Pelvic Pain: Christin Veasley, IPRCC and Chris Mullins, NIDDK
For this analysis, chronic pelvic pain (CPP) encompasses vulvodynia, interstitial cystitis, endometriosis, and prostatitis, a subset of visceral pain. This grouping is based primarily on shared symptomatology (e.g. pain localized to the pelvic floor and/or urogenital region) and common hypotheses relating to underlying causes.
Overview of the Portfolio
The portfolio includes basic (48%), translational (11%), and clinical studies (41%). Multiple NIH Institutes contribute to the portfolio through training/career awards, center grants, research project grants and a multi-site cooperative agreement. Fifty percent of the grants focus on interstitial cystitis, 23% on nonspecific pelvic pain, 14% on endometriosis, 9% on vulvodynia, 4.5% on IBS, and 2% on prostatitis. Overall, there is an emphasis on mechanisms of nociception and pain (23%), with a large percentage of this group focused on neuronal plasticity/peripheral sensitization, spinal cord processing, and inflammatory hyperalgesia. Additional areas emphasized include development and validation of pain models (15%), epidemiological studies (8%), and studies of chronic overlapping pain conditions (8%). These areas are justified based on current knowledge of CPP.
Gaps
- Characterization of bio-behavioral and psychosocial mechanisms that influence the experience, management, and morbidity of pain, and evaluation of treatment strategies targeting these mechanisms
- Development of tools for better assessments of symptoms (e.g. Quality of Life (QoL), physical and emotional function, both) for use in clinical studies and informing treatment strategies
- Identification of factors/mechanisms underlying acute urogenital/pelvic incidents turning chronic, disease progression, and resolution, for treatment and prevention of chronic symptoms
- Development and validation of outcome measures that assess multiple domains (e.g. pain severity and interference, psychosocial sexual functioning, sleep, mood), to study the efficacy and mechanisms of non-pharmacological treatments and inform treatment decisions
- Analgesic development and medical management, including team-based and self-management
Commonalities with Other Pain Conditions
Animal models exist for CPP, but the field would benefit from a standardized and prioritized approach to model validation and increased emphasis on modeling symptomatology relevant to the clinical condition.
Shared Interests and Opportunities to Collaborate
A number of highly sophisticated efforts addressing pain-centered syndromes using novel approaches were recently developed for CPP and related pain fields. They include active cooperative research studies, such as the OPPERA study, the Program Project Complex Persistent Pain Conditions: Unique and Shared Pathways of Vulnerability, and the MAPP Research Network, as well as efforts to provide improved guidance and resources to support future research and clinical pain-related trials, such as IMMPACT and numerous ACTTION initiatives.
Osteoarthritis: Ron Dubner, IPRCC
Osteoarthritis of the knee alone is a major chronic pain condition affecting 12% of the U.S. population over 25, and 30% of the population over 60 years of age.
Overview of the Portfolio
The osteoarthritis portfolio predominately comprises clinical studies and has a large portion focused on peripheral tissue changes associated with local arthritis. The portfolio includes 104 grants of which 60% were categorized as clinical and 26% as basic or translational, with the remainder being resource support awards. The clinical grants primarily focus on physical activity and strength following surgery or morphological changes visualized with imaging techniques, with smaller numbers related to surgery, biobehavioral therapy, central nervous system mechanisms, sleep, acupuncture, and biochemical treatments. The grants categorized as basic or translational encompass three major categories: repair of peripheral tissues, cytokine local therapy, and peripheral tissue changes (mainly cartilage).
Gaps
- Basic and translational studies on CNS changes
- Basic and translational studies on disease chronicity
- Clinical studies on associated psychosocial traits
Peripheral Neuropathies: Tina Tockarshewsky, IPRCC and Linda Porter, NINDS
The peripheral neuropathies arise from various diseases processes and conditions that damage peripheral nerves. Studies in this analysis reflect the most common neuropathy patient populations. In the U.S., 15-18 million people have diabetic peripheral neuropathy, 500,000 each have HIV-neuropathy and chemotherapy-induced peripheral neuropathy (CIPN), and 125,000 have Charcot-Marie Tooth hereditary neuropathy.
Overview of the Portfolio
The portfolio includes 69 projects, most with a basic science component (48%), with fewer focused on translational (22%) and clinical research (29%). The basic science projects primarily focus on underlying mechanisms of neuropathy-induced pain, peripheral nervous system sensitization, and molecular targets for therapies. The mechanistic studies cover a range of disease or condition specific neuropathies, which is indicative of the unique mechanisms associated with different etiologies. Translational research is mostly development and validation of therapeutic approaches that target pain symptoms as well as disease processes (projects that focus on disease processes, but do not include pain-related studies, would fall outside this pain portfolio). Most clinical projects address diabetic neuropathy, post herpetic neuralgia (PHN) or treatment-induced neuropathies (e.g., CIPN) and include assessment of molecular, behavioral and adjunct therapies, and development of diagnostic tools. The greatest need in this area is disease-modifying agents, which is reflected in the range of basic science projects in the portfolio. The distribution of topics reflects the challenges and opportunities facing the peripheral neuropathy community.
Gaps
- moving molecular therapies targeted to disease products to clinical trials
- assessment of access to and use of healthcare and comparative effectiveness of treatments
- epidemiological data that includes access to care, educational opportunities
- development of preventive therapies
- unique populations, especially pediatric diabetes
- research training
Commonalities with Other Pain Conditions
Study findings on PN, especially those related to neuropathic pain mechanisms and peripheral nervous system hypersensitivity will benefit a wide range of research on other neuropathic pain conditions. Because neuropathies are associated with numerous disease as well as treatment processes, peripheral neuropathy projects addressing disease processes relate to conditions of interest to nearly all NIH institutes.
Shared Interests and Opportunities to Collaborate
Because neuropathic pain is the result of nerve damage from disease or trauma, projects seeking to address nerve repair, nerve regeneration, and neuroprotective models represent the greatest areas of opportunity for collaborative efforts aimed at preventing and treating many of these painful neuropathies (especially for toxic neuropathies like CIPN, HIV-neuropathy, and diabetes).
Multiple Chronic Pain Conditions: Ron Dubner, IPRCC
A group of frequently co-occurring pain conditions that involve deep tissues (mainly muscle, joint, and viscera) with underlying mechanisms that are poorly understood were analyzed. They include disorders referred to as overlapping pain conditions: low back pain (LBP), which affects about 40% of the population, irritable bowel syndrome (IBS), which affects 10-15% of the population, temporomandibular disorders (TMD) and orofacial pain, which affect about 6.5% of the population, and fibromyalgia (FM), which affects over 6% of the population.
Overview of the Portfolio
Chronic LBP includes 63 grants (17% basic, 8% translational and 75% clinical) with an emphasis on peripheral tissues. The basic science grants mostly are related to spinal loading, cartilage proteins, and CNS pain mechanisms. Clinical grants primarily cover physical therapy, psychotherapeutic interventions, and imaging.
IBS (49 grants: 33% basic, 6% translational, 61% clinical): Basic science studies are mainly related to peripheral hypersensitivity and stress, with a few on CNS pain mechanisms. Translational studies focus on probiotics. Clinical grants primarily are related to treatment approaches, behavioral studies, and brain imaging. Many of the grants focus on GI issues, with little attention on psychological factors and modulatory influences.
TMD and orofacial pain (44 grants: 66% basic, 2% translational, 32% clinical): The basic science grants address peripheral (28%) and central nervous system (36%) mechanisms, treatments (7%), imaging (4%), and regeneration and bioengineering (25%). These grants mostly are related to neural mechanisms and tissue regeneration. The clinical grants are focused mainly on biobehavioral risk factors and treatments.
FM (19 grants: 16% basic, 5% translational, 68% clinical): Clinical grants are related to biobehavioral studies, treatments, risk factors, and acupuncture studies.
Gaps
- Relevant basic science mechanistic studies, other than for TMJ and IBS grants
- LBP preclinical studies focused on CNS mechanisms
- Clinical LBP studies related to psychosocial traits
- Studies of sensory mechanisms and the transition from acute to chronic pain for TMD
Shared Interests and Opportunities to Collaborate
There should be collaboration, especially for basic and translational studies, where overlap among the conditions is prominent including: 1) relevant preclinical animal studies and the transition from acute to chronic pain; 2) CNS mechanisms of pain amplification common to the conditions; 3) central mechanisms that lead to modulatory influences on the sensory component of pain experience; 4) common psychosocial risk factors; 5) the relationship of psychosocial risk factors to forebrain activity revealed by brain imaging; 6) common gender, genetic, and epigenetic risk factors.
Cross Section of Selected Conditions - Trauma, Headache, and Neuropathic Pain: Audrey Kusiak, IPRCC
This portfolio analysis addressed a combined set of topics to allow categorization according to the primary condition (e.g. chronic low back pain, cancer/chemotherapy, headaches, etc.), co-existing conditions (e.g. pain, PTSD, depression), concurrent themes (e.g. genes, techniques, drugs, etc.), experimental approach (e.g. imaging, clinical trial, genetics), and overlap with other pain conditions.
Overview of the Portfolio
This combined conditions portfolio consisted of 295 projects funded by DoD, NIH, and/or the VA. The primary conditions included were disease related (41%: e.g. diabetes, headache), nerve and inflammation related (20%), trauma related (17%: e.g. surgery, amputation), therapy related (5%: chemotherapy, HIV treatment), or focused on modulation by psychological condition (2%: e.g. mood disorders). Twenty two percent of the projects examined topics relevant to co-existing conditions, including multiple painful conditions (6%), mental health conditions (mood disorders, PTSD/anxiety; 9%) and substance abuse (2%), and sleep disorders (2%). Experimental approaches used in these studies were broad ranging and included mechanistic (36%), pharmacological (6%), non-pharmacological (3%), bio-behavioral and adjunct therapies (11%), genetic (5%), risk assessment (9%), and clinical trials (11%), as well as others.
Gaps
- Co-existing chronic pain and mental health studies
- Gender, age, and racial differences in pain perception and disparities in the treatment of pain
- Prevention of the transition from acute to chronic pain
- Headache and traumatic brain injury
Shared Interests and Opportunities to Collaborate
Within these selected portfolios, there are many areas of mutual interest across the Federal agencies including: use and addiction associated with opiates, the impact of co-existing mental health conditions on pain perception and pain care, the study of complementary and alternative approaches to treat chronic painful conditions, and the transition from acute to chronic pain. Numerous pain conditions are also of shared interest between a number of agencies, for example, headache, genotyping and phenotyping of pain conditions, nerve pain from trauma, and musculoskeletal pain conditions.
Conclusions from the Analyses of Selected Pain Conditions
The selected set of conditions highlights the breadth of areas covered and identifies opportunities for future research that are relevant to all pain conditions within the federal portfolio. Many of the identified research opportunities and gaps are unique to each condition, but several gap areas span the set of conditions in the analysis. Current phenotyping tools and procedures, especially as relate to bio-behavioral and psychosocial traits are identified as insufficient across the conditions. Genotyping and knowledge of genetic mechanisms, considered an area of promise to advance pain research, also are noted as gaps that span the portfolios. Risk factors and mechanisms that drive the transition from acute to chronic pain are considered as areas in need of further research as is development of targeted analgesics.
Although pain conditions have many unique etiologies and characteristics, there are several areas of study identified in particular portfolios that will benefit a wide range of other chronic pain conditions. The analyses identified several such areas. One area represented in the basic research portfolio, the contributions of central nervous system sensitization to inducing (transition from acute to chronic pain) and maintaining chronic pain is relevant to many pain conditions and to the co-existence of multiple chronic pain conditions in an individual. It also was noted that behavioral models and symptom-based models are adaptable across research programs on many pain conditions.
Pain Research Advances
A complementary charge to the IPRCC in the Patient Protection and Affordable Care Act (PPACA) is to develop a summary of most important advances in pain care research supported by Federal funding, relevant to the diagnosis, prevention, and treatment of pain and diseases and disorders associated with pain. To this end, the IPRCC selected 23 advances that exemplify the complex nature of pain and the range of research approaches that is crucial to moving the field forward. They include peer reviewed manuscripts, clinical studies, and collaborative projects within the following themes: Molecular Advances, Therapeutics, Systems Neuroscience, Specific Conditions, Pain in Special Populations, and Population-Based studies. The IPRCC will highlight important new advances on an annual basis, along with seminal articles in pain research that had a significant impact on the field. These advances are posted on IPRCC website. They highlight the state of the science in pain research and provide insight into future studies that will advance the field. The research areas chosen represent active areas in pain research and are summarized below.
Molecular Advances highlight basic research on understanding pain signaling mechanisms. A significant number of receptors and channels are thought to facilitate pain signaling, and pain therapies are developed through the targeting of these sites of action. These studies demonstrate that various signaling molecules, proteins, and inhibitors affect pain intensity in non-human model systems. For example, the compound naloxone was found to reverse chronic neuropathic pain in rats through inhibition of the Toll-like receptor 4, which regulates cells thought to be critically involved in the development and maintenance of chronic pain. A large number of federal pain grants fall within the research area of Pain Mechanisms, a broad research theme in this portfolio analysis.
Pain research investigators have demonstrated that certain populations experience pain differently and may be more vulnerable to pain. Additional barriers, such as access to healthcare and inadequate treatment, may exist for these populations. The Pain in Special Populations advances highlight research in diverse populations, including women, racial and ethnic groups, and the elderly. These studies characterize pain levels, pain treatment, and pain care for vulnerable populations. One selected research advance was a review published by Fillingim et al. on sex-related influences on pain. The study found that the most common forms of pain, including musculoskeletal and neuropathic pain, are more prevalent among women. Gender roles, cognitive factors, and hormonal influences may contribute to sex differences in pain and response to treatment. Disparities research is a cross-cutting theme within the pain research portfolio that overlaps with other research areas, such as Pain Mechanisms and Training & Education.
Large multidisciplinary population-based studies are crucial for our understanding of the prevalence, incidence, and causes of pain conditions. The Population-Based advances highlight large studies that generally focus on a major pain condition with the potential for sub-analyses of comorbid or overlapping pain conditions. The Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study was designed to test a candidate panel of genes and identify the physiological, psychological, and clinical factors causing first onset Temporomandibular Joint Disorders. Researchers discovered that individuals with a higher frequency of other overlapping pain conditions were more likely to have chronic TMJ and increased psychological stress and heightened self-awareness of pain. Thus, a follow-up study will include aims to identify risk factors that predict whether TMJ develops as a single condition or in conjunction with other pain conditions, including headache, low back pain, irritable bowel syndrome, and widespread bodily pain. These large studies encompass a number of overarching themes, such as Pain Mechanisms, Risk Factors & Causes, Surveillance & Human Trials, Disparities, and Overlapping Conditions.
Specific Conditions advances highlight pain research on a particular pain condition or set of conditions. Pain disorders may be specific to a body site, such as osteoarthritis, or cancer pain, or vulvodynia show more prevalence in a particular population, such as sickle cell pain, but chronic pain shares commonalities across conditions. Although pain conditions often have distinct characteristics, a new area of pain research has focused on identifying underlying mechanisms that are shared across conditions. A selected science advance by Reed et al., demonstrated that women who reported having irritable bowel syndrome, fibromyalgia, or interstitial cystitis were more likely to screen positive for vulvodynia. These types of studies highlight the importance of research on overlapping conditions.
Systems Neuroscience is a crucial field of research to understand how pain is processed by the brain and peripheral nervous system. The development of tools and instruments to diagnose and define pain conditions, and accurately measure pain in an objective manner relies on systems neuroscience. Brain imaging noninvasively measures brain activity that is closely associated with physiologic events. Imaging coupled with a machine learning algorithm tool allows researchers to develop pain models in which known stimuli can be used to test novel stimuli. Such a study was performed by Dr. Sean Mackey’s lab in which brain scans were captured during application of varying heat intensities applied to the forearm. The tested individuals were asked to self-report whether the stimuli were painful or not painful according to a scale. Previously untested individuals were subsequently subjected to the same stimuli and the trained machine was able to distinguish whether the stimuli were painful or non-painful based on brain scans alone. This study demonstrates the utility of brain imaging and machine learning for assessing pain levels without requiring any communication from the person.
Animal and human pain models are necessary to bridge the gap from basic to clinical research. Pain research on therapeutics often focuses on the development of such models, as well as the development of device and therapy delivery systems, and analgesics. The development of animal models is particularly important when common analgesics are not effective for a given pain condition. Neuropathic pain, for example, is not readily treatable by common pain medication, including opioids. A selected research study within the Therapeutics advances used a mouse model of spinal cord injury to demonstrate that transplantation of GABAergic neurons reduced inflammatory pain in mice. The proposed mechanism by which GABAergic neurons function to reduce pain is by inhibiting the over-activation of excitatory neurons, which may cause persistent pain.
Overall Summary and Conclusions
The Federal Pain Research Portfolio Analysis was undertaken to facilitate the tasks directed by the PPACA: to identify critical gaps in basic and clinical research on the symptoms and causes of pain, and make recommendations to ensure that the activities of the National Institutes of Health and other Federal agencies are free of unnecessary duplication of effort. The analysis covered more than 1200 research projects across six federal agencies and departments. These projects represent nearly $430,000,000 in pain research funding for 2011.
The portfolio covers a broad range of research topics from basic molecular mechanisms of pain to effectiveness and quality of health care for people with chronic pain. Its breadth and depth provides a wealth of opportunity for enhanced collaboration across the Federal Agencies and Departments that have an interest in pain. The analysis revealed many areas of shared research interests between and across Federal entities, but no notable redundancies within these topic areas. A review of the projects within specific research areas supported by multiple Federal entities revealed many complementary projects, but none for which spending or efforts were duplicated. The diversity of research within a focused topic area reflects each Agency’s or Department’s support of research most relevant to its unique mission and patient population. Partnerships between Federal funding agencies have developed to leverage the research resources and data generated in some areas of shared interests. The PASTOR-PROMIS initiative is an example of ongoing collaborative efforts where significant funds and effort from multiple sources have been committed to the project. Other areas of shared interests emerged from the analysis and have yet to benefit from trans-agency collaboration to enhance the research efforts, although planning efforts for some areas are ongoing. Whereas, the Federal pain research portfolio appears to be free of unnecessary duplication of effort, there are many areas of shared research interests that present opportunities to coordinate and leverage funding resources through increased collaboration at the level of the funding entity and the investigators.
Identification of gaps in basic and clinical research on causes and symptoms of pain was approached by first sampling detailed portions of the portfolio. Analysis of the selected set of pain conditions revealed needs for enhanced research that are unique to specific chronic pain conditions and some that are relevant to all pain conditions. The research gaps that cut across pain conditions, to some extent are those that also have emerged as promising areas to advance pain research, such as genotyping and genetic underpinnings of pain mechanisms and discovery of risk factors and mechanisms that underlie and predict the transition from acute to chronic pain.
The detailed categorization of all Federal pain research projects highlighted recent advances and emerging research that promise to move the field forward. It also identified areas for which the broad and multidisciplinary research portfolio provides opportunities for further research to address unanswered questions. The multidisciplinary nature of the portfolio is revealed in the overlap analysis between thematic and topic areas. This important aspect of the portfolio addresses the complex mechanisms of chronic pain, the multifaceted risk factors for developing pain, and the approaches to be considered for individual treatment strategies, as well as health care delivery to optimize pain management. Further opportunities exist to enhance interdisciplinary research however, especially through collaboration with investigators outside the area of pain. Relevant research on mechanisms in epilepsy and learning and memory may help to understand central nervous system changes associated with pain and provide targets for novel analgesics. The rapidly emerging field of altered brain function from trauma provides an opportunity to explore the most commonly associated conditions of PTSD, chronic pain including headache. The expanding field of research on management and treatment outcomes of multiple chronic conditions provides an opportunity for inclusion of chronic pain in relevant programs. Expansion of collaborative research efforts to fields relevant to pain may provide new approaches to understanding and treating pain.
Although a large proportion of the portfolio is basic research, we still do not understand fully the mechanisms by which pain evolves to a chronic state. This knowledge is important not only to better diagnose and develop treatments for single pain conditions, but also to determine why multiple pain conditions exist in an individual. One promising area of basic research focused on central nervous system sensitization may help to determine the mechanisms for co-occurrence of multiple chronic pain conditions, but other avenues such as neuroimmune interactions, need to be explored. Development of phenotyping and assessment tools that incorporate biopsychosocial components of pain for single and overlapping pain conditions also will provide better research tools to address this question and help to inform approaches to care.
Translational research links basic research findings to the clinic and is therefore, an area of recognized importance. Overall, the translational research portfolio is the smallest of the three Tier 1 categories. Although it has increased relative to basic and clinical research (9-13% of the portfolio from 2002 to 2011), there remain opportunities to enhance this area of research. The development of more efficient screening tools and approaches provides opportunities to bring needed novel pharmacological and non-pharmacological therapies into clinical evaluation. The importance of reverse translational research, applying clinical findings to direct basic research, has been recognized by some in the pain research field and is an area that may provide valuable opportunities to advance the field.
Clinical assessment tools for pain and outcome measures of pain management are not consistent across the research realm or in clinic practice. Development of consistent and meaningful instruments for individual patients and for population-level surveillance studies will generate quality data to further research and to inform care.
Population level studies on quality of care and access to care, and the disparities that exist among different groups in obtaining appropriate pain management are of particular relevance with the changing health care environment.
The current analysis of the Federal pain research portfolio is based on an extensive review of projects supported in a selected time frame. It provides a basis for future trends in pain research advances and an important tool to assist in directing and sharing resources to enhance the research effort.
Appendices
Federal Agency Missions
Agency for Healthcare Research and Quality (AHRQ):
The Agency for Healthcare Research and Quality's (AHRQ) mission is to improve the quality, safety, efficiency, and effectiveness of health care for all Americans. As 1 of 12 agencies within the Department of Health and Human Services, AHRQ supports research that helps people make more informed decisions and improves the quality of health care services.
Centers for Disease Control and Prevention (CDC):
CDC works 24/7 to protect America from health, safety and security threats, both foreign and in the U.S. Whether diseases start at home or abroad, are chronic or acute, curable or preventable, human error or deliberate attack, CDC fights disease and supports communities and citizens to do the same. CDC increases the health security of our nation. As the nation’s health protection agency, CDC saves lives and protects people from health threats. To accomplish our mission, CDC conducts critical science and provides health information that protects our nation against expensive and dangerous health threats, and responds when these arise.
Department of Defense (DoD):
The mission of the Department of Defense is to provide the military forces needed to deter war and to protect the security of our country. The department's headquarters is at the Pentagon.
U.S. Food and Drug Administration (FDA):
FDA is responsible for protecting the public health by assuring the safety, efficacy and security of human and veterinary drugs, biological products, medical devices, our nation’s food supply, cosmetics, and products that emit radiation.
National Institutes of Health (NIH):
NIH’s mission is to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce illness and disability.
U.S. Department of Veterans Affairs (VA):
To fulfill President Lincoln's promise “To care for him who shall have borne the battle, and for his widow, and his orphan” by serving and honoring the men and women who are America’s veterans.
Tier 1 Definitions
Basic Research:
Basic research is the systematic study of the fundamental aspects of phenomena and of observable facts without specific development of processes, products or clinical applications. Projects typically include studies of the mechanisms of normal or disease related processes at the molecular, cellular, systems or organ level.
Translational Research:
Translational research is the process of developing ideas, insights, and discoveries generated through basic scientific inquiry for the treatment or prevention of human disease.
Clinical Research:
Patient-oriented research. Research conducted with human subjects (or on material of human origin such as tissues, specimens and cognitive phenomena) for which an investigator directly interacts with human subjects. Excluded from this definition are in vitro studies that utilize human tissues that cannot be linked to a living individual. Patient-oriented research typically includes therapeutic interventions and applications of new technologies, clinical trials, epidemiologic and behavioral studies, outcomes research and health services research.
Tier 2 Definitions
Analgesic Development:
Research where the main focus is on translation of research findings into the development of novel drugs for pain. Includes optimization of candidate molecules, target engagement, pK, toxicological, and preclinical efficacy studies that support IND criteria and clinical trials. Also includes development of high throughput drug screens.
Analgesic Drug Safety:
Includes preclinical and clinical studies on analgesic drug safety, adverse effects, and surveillance. Also includes research on off-label use of medications for pain, and effects of direct to patient marketing.
Biobehavioral & Psychosocial Mechanisms:
Includes approaches to understanding how behavioral factors contribute to pain and how the use of behavioral/cognitive conditioning methodology can lessen the impact of pain. Also includes the impact pain has on behavior. Psychosocial mechanisms and treatments includes approaches to understanding pain in the context of a person’s social context (expectations, perceptions, thoughts and beliefs, reactions to pain, and response to treatments) and psychological state (affective, emotional influences). Also includes evaluation of treatment approaches that address or apply psychosocial factors to pain management.
Chronic Overlapping Conditions:
Includes shared biopsychosocial mechanisms underlying overlapping pain conditions, treatment approaches and population studies of these conditions.
Comparative Effectiveness Research:
Includes studies designed to compare the effects (benefits and harms) of methods to prevent, diagnose, treat, and monitor pain (medical, behavioral, device or CAM therapies). Studies may target existing or novel therapies, drug adverse events at the individual or population level. Also includes studies to compare models of care delivery systems and providers of pain therapies and procedures (relates to certification of health care providers for procedures and therapies. Also includes studies using registries or records from in a longitudinal manner to determine relative effectiveness of treatments and new methods of gathering such data.
Development of Animal & Human Models:
Includes development of novel preclinical models of pain conditions, genetic models, new behavioral measures of pain in animal, translational models, and novel assays for nociception and pain in humans.
Device & Therapy Delivery Systems Development:
Research where the main focus is on translation of research findings into the development of novel devices or therapy (drug, non-drug) delivery systems for pain management. Includes studies for development, refinement, and evaluation of devices and delivery systems that support criteria for IDE and clinical trials.
Diagnosis & Case Definitions:
Includes development of new methodology, tools, questionnaires, and procedures for developing and performing diagnostics and adaptations of clinical case definitions of pain as tools for population research.
Epidemiology:
Includes retrospective and prospective population studies of pain and pain disorders across the lifespan. Includes prevalence and incidence of pain conditions and related variables such as burden quality of life, occupational, family and societal impact, co-occurring disorders, substance abuse, and research related to case definition of pain and management measures.
Genetics and Genomics:
Includes genome-wide approaches, candidate gene studies, epigenomics, heritability, functional genomics, pharmacogenomics as related to nociception and pain and as risk or protective factors for chronic pain.
Health Care Utilization:
Includes dissemination and implementation of health care, structural barriers to pain, and management of transitions in clinical settings/utilization of different health care settings for patient care.
Health Disparities & Access to Care:
Includes studies on disparities in pain and pain disorders, access to care, and quality of pain management for ethnic and racial minorities, and socio-economic status.
Informatics, Databases, & IT Development:
Includes studies to develop and validate tools for population studies and for data acquisition to support future pain research. Includes development of registries and databases.
Mechanisms of Transition Phases:
Includes studies specifically focused on understanding the mechanisms and contributing factors that cause acute pain to become chronic and chronic pain to resolve. Includes mechanisms of CNS and PNS maladaptive neuroplasticity as they relate to these transitions. Also includes development and evaluation of prevention and treatment strategies that target transition phases.
Medical Management:
Includes development, validation, and evaluation of self-management approaches to pain care with e-health and m-health technologies such as cell phones, EMA, websites, and other systems to train, encourage, and monitor patient behavior and compliance to treatment. Includes development, improvement, and evaluation of teambased approaches to pain management, through engagement of health care providers from multiple disciplines involved in patient care. Subcategories include: a) Self-management approaches and b) Team-based treatment approaches.
Neurobiological/Glial Mechanisms:
Includes studies of molecules, molecular and signaling pathways, cellular and cell-cell interaction mechanisms, neuro-immune systems interactions, central and peripheral mechanisms of hypersensitivity, CNS and PNS neuroplasticity.
Non-Pharm Mechanisms & Treatments:
Includes the mechanisms of analgesia underlying CAM, physical activity vs. sedenterism, fitness and exercise, procedural and surgical therapies. Also includes development, validation, evaluation, and management measures of these treatment approaches. NOTE: Excludes psychosocial and biobehavioral mechanisms and treatments, which are included in a different section.
Other “Omics”:
Includes any large-scale agnostic approaches including proteomics, metabolomics, kinomics, etc.
Outcomes & Health IT for Decision-Making:
Includes patient and provider reported outcomes; development and validation of pain rating scales, pain burden, disability, quality of life, and management measures.
Pain & Non-Pain Comorbidities:
Includes shared biopsychosocial mechanisms underlying comorbid pain and non-pain conditions (affective/behavioral and other medical conditions), treatment approaches and population studies of these conditions.
Pain & Trauma:
Includes mechanisms, therapy development, and management of pain caused by burn, peri-operative procedures, TBI, SCI, and other nerve injuries, and other traumatic events.
Pain Education:
Includes various means and methods to develop, validate, disseminate, and implement educational materials, programs, and curricula to better inform patients, care providers (caregivers, health care professionals), advocates, and the public. Subcategories include: a) Health care provider education; b) Caregiver education; c) Patient education; and d) Public Education
Pain Prevention:
Includes studies on approaches and measures of prevention, risk factors and susceptibility to pain at individual and population levels.
Pharm Mechanisms & Treatments:
Includes mechanisms of analgesia underlying pharmacological approaches; behavioral pharmacology, pK, studies of active metabolites, and drug-target (single and multiple) interactions. Also includes evaluation and management measures of these individual or combined (poly-pharmacy) treatments.
Sex & Gender Differences:
Includes research on sex and gender differences (males and females, and lesbian, bisexual, gay, and transgender in biopsychosocial mechanisms and treatments for pain and pain conditions, susceptibility to pain, and treatment response. Subcategories include: a) Male and b) Female
Substance Use & Abuse/Addiction:
Includes research on pain and pain management in populations with abuse/addictive behaviors and abuse and addiction prevention and management. Also includes studies on shared biopsychosocial mechanisms of reward mechanisms and pain.
Training in Pain Research:
Training in pain research at graduate, post graduate levels, and for health care providers (includes mechanisms such as NIH K, F, T grants).
Unique Populations:
Includes research on pain and pain conditions that target unique study populations including pediatric, elderly, end of life, disabled, and military. Subcategories include: a) Pediatric; b) Elderly; c) End of Life; d) Disabled; e) Military
Women’s & Minority Health Research:
Includes studies that focus on women’s health issues and minority health issues as regards pain conditions and management of pain conditions. Note that research on health disparities should be listed in #17. Subcategories include: a) women.
Detailed Analysis of Tier 2 category: Neurobiological and Glial Mechanisms of Nociception
Twenty percent of the Federal pain portfolio was coded as basic research on neurobiological and glial mechanisms of nociception (Tier 2 category definition). This set of projects includes a broad range of basic research topics. IPRCC members, Dr. Michael Moskowitz and Dr. Ronald Dubner performed a more detailed analysis of this category by coding the grants into subsets of pain research defined by the Society for Neuroscience.
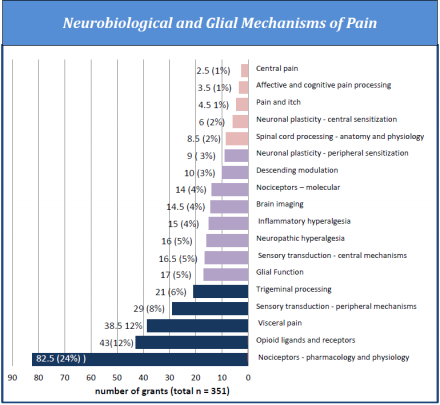
The chart shows the distribution of grants in the Tier 2 category, neurobiological and glial mechanisms of nociception, according to the Society for Neuroscience classifications for pain research.
Grouped Pain Conditions
Neuropathic Pain
Peripheral Neuropathies
Central Pain
Phantom Pain
SCI Pain
Sciatica
Chronic Regional Pain Syndrome
Neuropathic Pain (non-specific)
Neuropathic Cancer Pain
Visceral (GI) Pain
Irritable Bowel Syndrome
Visceral Pain
Peripheral Neuropathies
Peripheral Neuropathies
Painful Diabetic Neuropathy
HIV AIDS painful
Post Herpetic Neuralgia Treatment-induced pain
Charcot Marie Tooth
Carpal Tunnel and other
Acute/Subacute Pain
Procedural Pain
Burn Pain
Trauma Pain
Peri & post-operative Pain
TMJ/Orofacial Pain
TMJD
Dental and Intra-oral Pain
Orofacial Pain (neural and muscular)
Chronic Pelvic Pain
Vulvodynia
Interstitial Cystitis
Endometriosis
Pelvic Pain (nonspecific)
Musculoskeletal Pain
Neck Pain
Other musculoskeletal pain
Other
Labor Pain
Chronic Fatigue Syndrome
Cardiac Pain
Medication-induced pain
Ocular Pain
Training in Pain anesthesia
Pain and Sleep Disturbance
Single Conditions
Osteoarthritis
Cancer Pain
Low Back Pain
Headache
Rheumatic
Sickle Cell Pain
Fibromyalgia
IPRCC Committee Member Roster (October 2012)
Chairperson
Landis, Story C., PhD
Director
National Institute of Neurological Disorders and Stroke
National Institutes of Health
Bethesda, MD 20892
Christopher, Myra J
Kathleen M. Foley
Chair for Pain and Palliative Care Center for Practical Bioethics
Kansas City, MO 64105-2116
Cowley, Terrie
President and Co-Founder
The TMJ Association
Milwaukee, WI 53226-0770
Dubner, Ronald, PhD, DDS
Professor
Department of Neural and Pain Sciences
University of Maryland Dental School
Baltimore, MD 21201
Gilbertson, Elizabeth B
Chief of Strategy
Unite Here Health
Aurora, IL 60598
Green, Carmen Renee', MD
Associate Vice President and Associate Dean for Health Equity and Inclusion
Professor of Anesthesiology, Obstetrics and Gynecology and Health Management and Policy
Schools of Medicine and Public Health
Office for Health Equity and Inclusion
University of Michigan Health System
Ann Arbor, MI 48109-5624
Helmick, Charles G. III, MD
Senior Medical Epidemiologist
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control And Prevention
Atlanta, GA 30341-3724
Kusiak, Audrey N, PhD
Scientific Program Manager
Office of Research Development
Rehabilitation Research and Development Service
Department Of Veterans Affairs
Washington, DC 20420
Mackey, Sean C, PhD, MD
Chief, Division of Pain Management
Associate Professor
Department of Anesthesiology
School of Medicine
Stanford University
Stanford, CA 94305
Miaskowski, Christine A., PhD, RN
Professor and Chair Department of Physiological Nursing
School of Nursing University of California, San Francisco
San Francisco, CA 94143
Moskowitz, Michael A., MD
Professor of Neurology
Harvard-MIT Division of Health Science And Technology
Harvard Medical School
Boston, MA 02115
Rappaport, Bob A., MD
Director
Division of Anesthesia, Analgesia and Addiction Products
Center for Drug Evaluation and Research
Food and Drug Administration
Silver Spring, MD 20993
Ricciardi, Richard, NP, FAANP, PhD
Health Scientist
Center for Primary Care, Prevention, and Clinical Partnerships
Agency for Healthcare Research and Quality
Rockville, MD 20850
Smith, Wally R, MD
Professor and Vice-Chair
Division of General Internal Medicine
Virginia Commonwealth University
Richmond, VA 23298-0306
Somerman, Martha J, PhD, DDS
Director
National Institute of Dental and Craniofacial Research
National Institutes of Health
Bethesda, MD 20892
Thomas, Richard W., MD, DDS
Major General, USA
Chief Medical Officer
Defense Health Agency
Falls Church, VA 22042
Tockarshewsky, Tina M.*
President and CEO
The Neuropathy Association
New York, NY 10018
Vargas, Mary Caroline, JD
Partner
Stein & Vargas, LLP
Emmitsburg, MD 21717
*Current IPRCC Member that contributed to the report
Veasley, Christin L.
Co-Founder
Chronic Pain Research Alliance
North Kingstown, RI 02852
